e) Using the half reaction below sHE Pou3- HPO,2 + 2H* + 2e HPO+H;O E--0,234 V and acid dissociation constants for H3PO4 (K=7.11 × 10°; K2 = 6.32 × 10*, K7.1 x 1014), calculate standard reduction potential for the half reaction he Oy H2POª + H* + 2e HPO + H;O
e) Using the half reaction below sHE Pou3- HPO,2 + 2H* + 2e HPO+H;O E--0,234 V and acid dissociation constants for H3PO4 (K=7.11 × 10°; K2 = 6.32 × 10*, K7.1 x 1014), calculate standard reduction potential for the half reaction he Oy H2POª + H* + 2e HPO + H;O
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 88E: For the following half-reaction, = 2.07 V: A1F63(aq)+3eAl(s)+6F(aq) Using data from Table 17-1,...
Related questions
Question

Transcribed Image Text:e) Using the half reaction below
sHE
Pou3-
HPO,2 + 2H* + 2e
HPO+H;O E--0,234 V
and acid dissociation constants for H3PO4 (K=7.11 × 10°; K2 = 6.32 × 10*, K7.1
x 1014), calculate standard reduction potential for the half reaction
he Oy
H2POª + H* + 2e
HPO + H;O
Expert Solution
Step 1
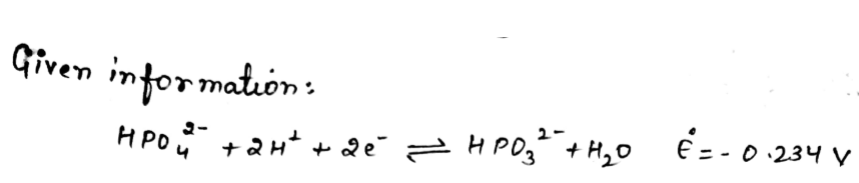
Step 2
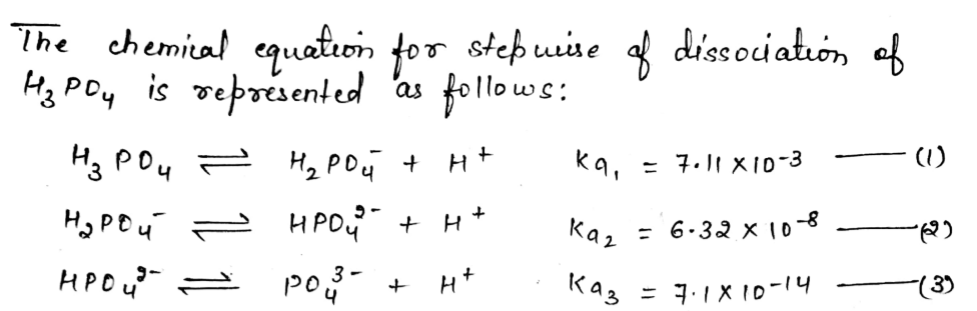
Step 3
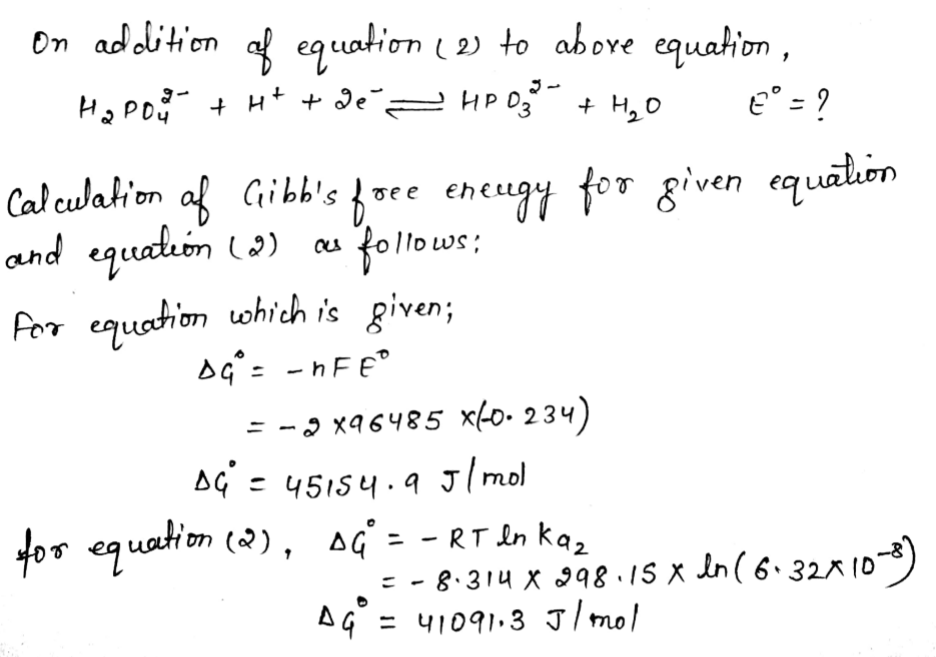
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning