
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Q
awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNsikr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHIntrzjVfiaJ_cKvcyqWTUEIAKXtcoqhtE9...
Gibbs Free E... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le... Math 115 W-S Fall..
18.3 Gibbs Free E... 5.3 Enthalpies of...
5.3 Enthalpies of... 18.5 Gibbs Free E...
18.5
20,449
2
E
W
S
ENTROPY AND FREE ENERGY
Calculating dG from dH and dS
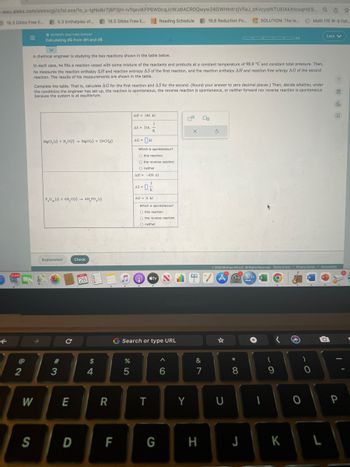
A chemical engineer is studying the two reactions shown in the table below.
In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 98.0 °C and constant total pressure. Then,
he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free erfergy AG of the second
reaction. The results of his measurements are shown in the table.
Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under
the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous
because the system is at equilibrium.
MgCl, (s) + H₂O() MgO(s) + 2HC1(e)
PO () + 6H₂O(1) 4H, PO ()
Explanation
#3
с
E
D
Check
NOV
26
$
4
R
.
F
AH 140, kJ
%
5
AS 316.
AG = KJ
J
K
Which is spontaneous?
O this reaction
O the reverse reaction.
O neither
AH = -439. kJ
J
AS = 0²/2
-0₁
AG= 0. kJ
Which is spontaneous?
O this reaction
O the reverse reaction
O neither
.
G Search or type URL
T
tv N
6
Y
X
Do
&
7
H
Ś
O▬▬OD 0/5
U
* 00
8
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Accessibility
"/A
0
0
1
(
9
JK
)
0
W
Lara V
0
?
db
A
P
T
L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each chemical reaction listed in the first column of the table below, predict the sign (positive or negative) of the reaction entropy ASxn: If it's not possible to decide with the information given, check the "not enough information" button in the last column. Note for advanced students: Assume the temperature remains constant. Assume all gases and solutions are ideal. reaction sign of reaction entropy AS rxn く0 H,so,(1) + H,0 (1) → H,0" (aq) + HSo, (aq) AS > 0 rxn not enough information. AS く0 rxn 4Fe(s) + 30, (g) → 2Fe,O,(s) AS > 0 rxn not enough information. AS 0 AS rxn not enough information.arrow_forwardIn each , she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 83.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction andAS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. AH = -1237, kJ AS = -3507. K 6C (s) + 6H, (g) + 30,(g) → C,H,o(6) AG = Which is spontaneous? O this reaction the reverse reaction O neither AH = -1648. kJ AS = AG = 0. kJ 4Fe(s) + 30, (g) → 2Fe,0,(s) Which is spontaneous? this reaction O the reverse reaction O…arrow_forwardFor the reaction 2 PbO (s) + 2 SO2 (g) 2 PbS (s) + 3 O2 (g), it can be calculated from AG°F values that AG°298 = %3D +780.8 kJ. What is AG (in kJ) at 298 K when the pressures of the gases are P(SO2) = 120 atm and P(O2) = 3.1 x 107 atm? O - 916 + 916 + 732 O + 722 O + 646arrow_forward
- A chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 91.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. AH = 439. kJ J AS = 1206. K AG = || kJ 4H,PO,(s) → P,0,10 (s) + 6H,0(1) Which is spontaneous? this reaction the reverse reaction neither AH = -803. kJ J AS = K AG = -4. kJ CH, (3) +…arrow_forwardA chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 25.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. AH = 1005. kJ AS = 3281. K AG = 4PF, (g) + 10H, (3) - P (s) + 20HF (g) Which is spontaneous? O this reaction the reverse reaction O neither AH = 1648. kJ AS = AG = 0. kJ 2Fe, 0, (5)…arrow_forwardA chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 51.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. 6C(s) + 6H2(g) + 30, (g) C6H12O6 (5) AH=1237. kJ AS = -3816. K 2CO2(g) 4H.O(g) → 2CH, OH (g) + 302(g) AG= kJ Which is spontaneous? this reaction the reverse reaction neither AH =…arrow_forward
- 4PC13 (9) P4(g) + 6C₁₂ (g) → C₂H₂ (g) + 502(g) → 3CO2(g) + 3CO2(g) + 4H2O(1) AH 1207. kJ AS = 3078. J - K AG = kJ Which is spontaneous? this reaction the reverse reaction neither ΔΗ = - 2220. ΚΙ AS = K AG = 20. kJ Which is spontaneous? this reaction the reverse reaction neitherarrow_forwardDo not answer in image format. Maintain accuracy and quality in your answer. Answer completely.arrow_forwardIn each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 106.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. 4H, PO () PO () + 6H₂O(0) 6C(s) + 6H₂(e) + 30,(e) - CH₂0,6) Alf 439 kJ AS 1091 - k AG= J Which is spontaneous? this reaction the reverse reaction neither AH-1237 13 AS= AG=-14. U Which is spontaneous? this reaction the reverse reaction neitherarrow_forward
- At 298 K, this reaction to produce phosphoryl chloride has a standard enthalpy of A,H° = -620.2 kJ mol-¹. 2 PCI (g) + O₂(g) → 2 POCI3(1) The standard molar entropies, in J K-1 mol-1, for these species are: PC13(g) [308.5]; O2(g) [205.1]; POCI3(1) [215]. What is the standard Gibbs energy of reaction at 298 K? (Note: Some of these numbers have been changed from their actual values for the purpose of the exam.) (Determine answer in units of kJ/mol with one decimal place. However, do not enter units with your answer.)arrow_forwardA chemical engineer is studying the two reactions shown in the table below. In each case, she fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 106.0 °C and constant total pressure. Then, she measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of her measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. MgO (s) + 2HC1(g) → MgCl₂ (s) + H₂O (1) P₁(s) + 20HF (g) → - 4PF₂ (g) + 10H₂(g) AH-140. kJ AS 454. AG = OKJ Which is spontaneous? Othis reaction AS = K the reverse reaction O…arrow_forwardA chemical engineer is studying the two reactions shown in the table below. In each case, he fills a reaction vessel with some mixture of the reactants and products at a constant temperature of 20.0 °C and constant total pressure. Then, he measures the reaction enthalpy AH and reaction entropy AS of the first reaction, and the reaction enthalpy AH and reaction free energy AG of the second reaction. The results of his measurements are shown in the table. Complete the table. That is, calculate AG for the first reaction and AS for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontaneous, the reverse reaction is spontaneous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium. AH = - - 1212. kJ J AS 4174. K Ś ? AG = 4H₂O₂ (1) + PbS (s) PbSO4 (s) + 4H₂O (1) ☐ kJ Which is spontaneous? this reaction the reverse reaction neither - 1237. KJ 6C (s) + 6H₂(g) + 30₂…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY