
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
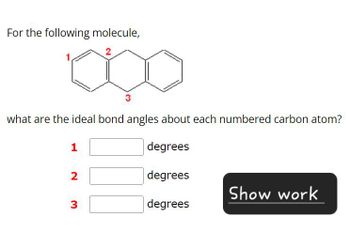
Transcribed Image Text:For the following molecule,
2
1.
what are the ideal bond angles about each numbered carbon atom?
1
degrees
2 3
degrees
Show work
degrees
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Indicate which of the following molecules are polar. Draw the molecular structure of each polar molecule, including the arrows that indicate the bond dipoles and the molecular dipole moment. (a) HCN (b) I2 (c) NOarrow_forwardConsider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, TF5, and SCl6. These 12 compounds are all examples of different molecular structures. Draw the Lewis structures for each and predict the molecular structures. Predict the bond angles and the polarity of each. (A polar molecule has a net dipole moment, while a nonpolar molecule does not.) See Exercises 25 and 26 for the molecular structures based on the trigonal bipyramid and the octahedral geometries.arrow_forwardAs a general rule, MX molecules (where M represents a central atom and X represents terminal atoms; n = 2 5) are polar if there is one or more lone pairs of electrons on M. NH3 (M = N, X = H, n = 3) is an example. There are two molecular structures with lone pairs that are exceptions to this rule. What are they?arrow_forward
- 1. Which of the following species has the longest N—O bond? NO3 NO+ NO2− H2NOHarrow_forwardWhy is it less clear to see the electrons on HBrO2 molecular electron geometry than it is on something like H2O? I know there should be 20 electrons on HBrO2, but this leads me to an incorrect drawing. And with the correct drawing, I don't see where all the electrons are. It seems like there's only 10 total. What am I missing here?arrow_forwardc. How many o and T bonds are there in acrylonitrile? H C=C2 a H 'N Acrylonitrile o bonds = T bonds =arrow_forward
- Can you please do the first 3 rows of the table?arrow_forwardMolecular geometry—don’t know why I’m incorrect all of a sudden. Might be one shape needed that is not given in the option choices.arrow_forwardTable 1.1 Molecular formula Lewis Structure SO, CO, 2 NO2 HCN CN- CIF; H;CO BF. :C=N: Total number of e groups around the central atom Number of lone pairs around the central atom 2 1 Electron geometry linear Molecular geometry Linear Approximate bond angles 180° Polarity poplar Table 1.1 continued Molecular formula Lewis Structure BeCl PFs SF4 H;S XeF2 SF. BrFs XeF4 Total number of e groups around the central atom Number of lone pairs around the central atom Electron geometry Molecular geometry Approximate bond angles Polarityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning