
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
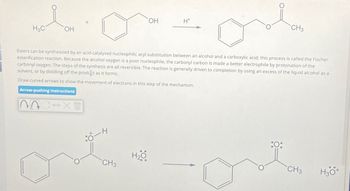
Transcribed Image Text:H3C
OH
OH
H+
CH3
Esters can be synthesized by an acid-catalyzed nucleophilic acyl substitution between an alcohol and a carboxylic acid; this process is called the Fischer
esterification reaction. Because the alcohol oxygen is a poor nucleophile, the carbonyl carbon is made a better electrophile by protonation of the
carbonyl oxygen. The steps of the synthesis are all reversible. The reaction is generally driven to completion by using an excess of the liquid alcohol as a
solvent, or by distilling off the product as it forms.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
CIX
10-4
H₂O
CH3
H₂O
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Carboxylic acids can be made by the hydrolysis of nitriles, which in turn can be made from an alkyl halide. Draw the structures of a starting alkyl bromide and the intermediate nitrile that would be used in the alkyl bromide nitrile isovaleric acid NC Н,о -> он Нarrow_forwardWhat is the source of the nucleophilic attack? How does the oxygen get protonated? What causes the elimination reaction?arrow_forward2-Hydroxybenzoic + Ethanoic anhydride. acid 15 minutes. 80 °C Sulfuric acid Products Ethanoic anhydride Ethanoic acid 2-Ethanoyloxybenzene carboxylic acid Benzoic acid Phenol 2-Hydroxybenzoic acidarrow_forward
- ОН Type of reaction 1-Propanol is heated Type of reaction H+, Heat IZ + t 1- NaOHarrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l). The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 8.50 gof butanoic acid and excess ethanol? Express your answer in grams to three significant figures.arrow_forwardIdentify the best reagents to complete the following reaction. HO, CIarrow_forward
- Can you draw the mechanism for the synthesis of cyclohexene from cyclohexanol through an acid-catalyzed dehydration reaction. Where Phosphoric acid donates a proton ((H^+)) to the hydroxyl group of cyclohexanol, forming a protonated cyclohexanol intermediate. • The protonated cyclohexanol undergoes dehydration, leading to the removal of a water molecule and the formation of cyclohexene. • The released proton combines with water to form hydronium ion (H3O+), regenerating the catalyst. The mechanism should illustrate the acid-catalyzed dehydration process, where phosphoric acid facilitates the removal of water from cyclohexanol, resulting in the formation of cyclohexene.arrow_forwardWhat is the role of phosphoric acid in the synthesis of cyclohexene? it is an antioxidant that prevents free radical side reactions it is a safe, non-toxic solvent it lowers the boiling point of the reaction mixture (a colligative property of adding phosphoric acid to water) it protonates the hydroxyl of cyclohexanol to make it a better leaving grouparrow_forwardH₂SO4, H₂O Choose the enol(s) that can be formed from the following oxymercration reaction. OH ОН OH HgSO4 ОНarrow_forward
- Give the systematic (IUPAC) names for these molecules. Boononon cnolonongron. НаСНз CHОССH2СH2СНCHЗ CH3 phenyl propanoate |4-methyl pentane methanoat Incorrect. You mixed up the acyl and alkoxy portions of the molecule. Name the alkoxy part first, followed by the acyl part.arrow_forwardMethyllithium (CH3Li) is often used as a base in organic reactions. Predict the products of the following acid-base reaction.CH3CH2¬OH + CH3¬Li ¡arrow_forward7 8 11 12 me CH3 H3C 3-butylpropanoate 2-butoxypropane propyl-2-butanoate 2-butylpropanoate H₂ C CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY