
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
answer everything pls and make sure it’s correct n clear
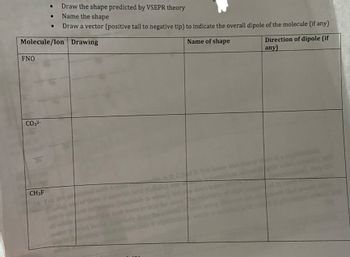
Transcribed Image Text:•
Draw the shape predicted by VSEPR theory
.
Name the shape
•
Draw a vector (positive tail to negative tip) to indicate the overall dipole of the molecule (if any)
Molecule/lon Drawing
Name of shape
Direction of dipole (if
any)
FNO
CO32-
CH3F
16 You are
look
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 17 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part carrow_forward%3D 1eg= lo00g 4. Perform each conversion. 3.55 kg to grams un. a)arrow_forward) STEC SHS STEMATC Q Search Quizlet » An iren A Classwork for GENERAL > Pick Up Lines for Weme D8 htps://dassroom google.comVzkzNaY2NEKAVV/all School-ing Oto weed O GOOGLE MEET O Grade 11- MNDLV DENTERTAINMENT Time -------> Sample Problems I O A 466-g sample of water is heated from 8.50 °C to 74.60 °C. Calculate the amount of heat absorbed (in kilojoules) by the water. O An iron bar of mass 869 g cools from 94 °C to 5 °C. Calculate the heat released (in kilojoules) by the metal. O How much energy is required to change 2600 gram of ice at 0°C into water at the same temperature? Sample Problems acer F5 F6 F8 F9 F10 F1 F12 CH Numk & 5 6 8. 9 T Y 4 F K 2 Larrow_forward
- See image belowarrow_forwardNeed help with homeworkarrow_forward45. | You find that if you hang a 1.25 kg weight from a vertical spring, it stretches 3.75 cm. (a) What is the force constant of this spring in N/m? (b) How much mass should you hang from the spring so it will stretch by 8.13 cm from its original, unstretched length?arrow_forward
- Chrome File Edit View History Bookmarks People Tab Window Help Hcc Dashbc x E Buy Es: x G find so x © Periodi x A ALEKS X HUc Chapte x E New m x G conver X 不→ C A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-IQiHqRdYV_6Ux63SypJXz0Coxvwqgg4JkWI7FgD9QGpr.. O GASES O OC D Jacqueline v Using Avogadro's Law Hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 7.5 m of nitrogen were consumed? Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. 圖 中 ロ alo oloarrow_forwardChrome File Edit View History Bookmarks People Tab Window Help * 30% (4) Sat 2:00 PM Q OE o Chem101 O General Chemistry I (LAB SCI) X + i app.101edu.co EApps MM Gmail YouTube 9 Maps A Translate https://www.carth. 9 Google Chrome isn't your default browser Set as default Question 7 of 7 Submit Using the equations 2 Sr(s) + O2 (g) → 2 Sro (s) AH° = -1184 kJ/mol CO2 (g) → C (s) + O2 (g) AH° = 394 kJ/mol kJ/mol Determine the enthalpy for the reaction C(s) + 2 SrO(s) → CO2 (g) + 2 Sr(s). 1 2 6 C 03.0 Se VHFORMA The MO -Standa 8 AH ma +/- x 100 8T國山電@ O etv Oct 24 MacBook Air 80 888 SC FS F6 F3 F2 %23 2$ & delete 3 5 8. W E R Y U H J к K ret F V M 4-arrow_forwarda .ull Asiacel| 10 N 141 N O A box with a volume (V=0.05 m³) lies at the bottom of a lake whose water has a density of (1*10³ kg/m³). How much force is required to lift the box, .if the mass of the box is (1000 kg) 9319.5 N O 9313.9 N O 9391.5 N O 9315.9 N O A future rectangular ship filled with oil. If the dimensions of the ship are (250 m) long, (80 m) wide, and (80 m) high. Determine how far the bottom of the ship is below the sea level? (Consider the total mass of the ship with the oil is (10.2*108 kg), and the .sea density is (1024 kg/m³))arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY