
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
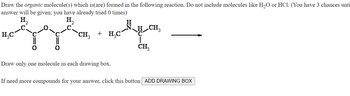
Transcribed Image Text:Draw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like H₂O or HCl. (You have 3 chances unti
answer will be given; you have already tried 0 times)
H₂
H₂
tyy nopm
CH₂
CH₂ + H₂C´
CH3
H₂C
Draw only one molecule in each drawing box.
If need more compounds for your answer, click this button ADD DRAWING BOX
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question Completion Status: QUESTION 2 Write a full balanced equation for the chemical reaction that occurs when an aqueous solution of potassium sulfide is mixed with an aqueous solution of sodium acetate. Give the state of each reactant and product (s, I, g, aq). тTT Arial 3 (12pt) T - Path: p Words:0 QUESTION 3 Ek Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answersarrow_forwardUse the down arrow on the far left of the row of icons above the space where you enter your equation to access the subscript "x2" function. If you cannot do this, then enter the subscripts on the same line, meaning I will accept formulas such as H2O. Use --> (dash dash greater than) for the arrow. Do not use the equals sign ("="). Write and balance the equation for the complete combustion of C4H10. You do not need to include physical states.arrow_forwardWhich one of the compounds in Figure #3 is the major organic product of Reaction #3? Reaction #3 Figure #3 CH₂ OH O compound A O compound B O compound C O compound D "C(CH₂) CH₂ C(CH3)3 compound A H₂SO4 (heat) Please click here if image does not display. CH₂ C(CH3)3 compound B CH₂ C(CH₂) compound C CH₂ "C(CH₂)s compound Darrow_forward
- Commercial concentrated hydrochloric acid has the following specification: Density = 1.2 g/mL; Weight percentage is 37% w/w: Molecular weight of HCl is 36.46 g/mol. If 12.0 mL of conc HC) is added to the reaction, how many moles of HCI have been added? Report your answer to the correct number of significant figures: do not report the units in your answer.OGMU 2020 QUESTION 3 If 5.0 mL of t-pentyl alcohol are involved in the reaction, how many moles of t-pentyl alcohol are present? Round your answer to the correct number of significant figures. Do not include the units in your answer. IUPAC name of t-pentyl alcohol is 2-methyl-2-butanol.OGMU 2020 (Hint: use the CRC Handbook Of Chemistry and Physics from our online library to get the correct numerical constants.) QUESTION 4 Given that the nucleophilic substitution reaction used 5.0 mL of t-pentyl alcohol and 12.0 mL of conc. hydrochloric acid to produce t-pentyl chloride, what was the limiting reagent in the overall reaction? GMU 2020 O…arrow_forward5. 'Н H H US LDA H₂O LDA H₂Oarrow_forwardPlease help ASAP, thank you! "Calculate the mass of diethyl ether (in grams) given the values listed in the reaction scheme. Keep your answer to 2 decimal places."arrow_forward
- Click to see additional instructions Balance this chemical equation with the smallest set of whole numbers. Enter a number for each compound. CH3CH2CH2OH (1) + |02 (g) |CO2 (g) + H2O (I)arrow_forwardCommercial concentrated hydrochloric acid has the following specification: Density = 1.2 g/mL; Weight percentage is 37% w/w: Molecular weight of HCl is 36.46 g/mol. If 12.0 mL of conc HC) is added to the reaction, how many moles of HCI have been added? Report your answer to the correct number of significant figures: do not report the units in your answer.OGMU 2020 QUESTION 3 If 5.0 mL of t-pentyl alcohol are involved in the reaction, how many moles of t-pentyl alcohol are present? Round your answer to the correct number of significant figures. Do not include the units in your answer. IUPAC name of t-pentyl alcohol is 2-methyl-2-butanol.OGMU 2020 (Hint: use the CRC Handbook Of Chemistry and Physics from our online library to get the correct numerical constants.) QUESTION 4 Given that the nucleophilic substitution reaction used 5.0 mL of t-pentyl alcohol and 12.0 mL of conc. hydrochloric acid to produce t-pentyl chloride, what was the limiting reagent in the overall reaction? GMU 2020 O…arrow_forwardEach step of a four-step reaction has a percent yield of 95%. What is the percent yield of the overall reaction? please show work.arrow_forward
- What reactant would transform the molecule from the top left to the top right. Given the same molecule, what would the given reactants transform it into below?arrow_forwardTry Again Row 3: Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A student runs two experiments with a constant-volume "bomb" calorimeter containing 1100. g of water (see sketch at right). First, a 7.000 g tablet of benzoic acid (CH₂CO₂H) is put into the "bomb" and burned completely in an excess of oxygen. (Benzoic acid is known to have a heat of combustion of 26.454 kJ/g.) The temperature of the water is observed to rise from 15.00 °C to 53.26 °C over a time of 12.3 minutes. Next, 5.420 g of acetylene (C₂H₂) are put into the "bomb" and similarly completely burned in an excess of oxygen. This time the temperature of the water rises from 15.00 °C to 74.42 °C. Use this information, and any other information you need from the ALEKS Data resource, to answer the questions below about this reaction: Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or…arrow_forwardIn each row check off the boxes that apply to the highlighted reactant. reaction 2+ → Zn (CH₂CN)² + (aq) 2+ Zn (aq) + 6 CH 3 CN (aq)- + Ag+ (aq) + 2 NH3(aq) → Ag (NH₂) (aq) 2 H₂(g) + 2 IC1 (g) → 2 HCl(g) + 1₂(g) The highlighted reactant acts as a... (check all that apply) 0 0 0 0 0 0 0 0 0 0 0 Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base Brønsted-Lowry acid Brønsted-Lowry base Lewis acid Lewis base X 5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY