
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help with all parts
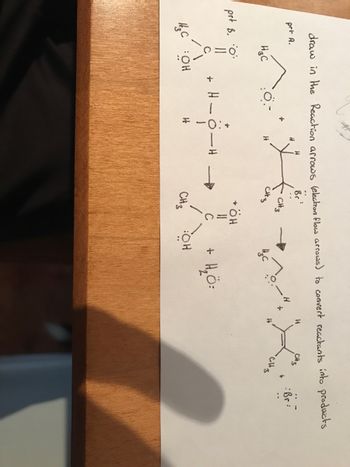
Transcribed Image Text:draw in the Reaction arrows (election flow arrows) to convert reactants into products
prt A.
Br
HC
prt B. 0:
HC OH
+
о-
H - O-
H
H
H
CH 3
CH3
+ ÖH
СН3
вон
нас
+ Н2 =
H
+
Н
H
Cit3
:Br:
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A high-throughput screen is performed to test compounds for anti-cancer activity. A compound is considered a hit only if it kills the cancer cells, but not normal cells. One test compound kills both the cancer and normal cells, and this is correctly detected by the assay. What kind of result is this? A false control result. A true negative result A false positive result. A false negative result.arrow_forward- tab s lock introl < STARTING AMOUNT esc Tap here or pull up for additional resources option ! 1 L A FI Q X N @ 2 W S X H # 3 80 F3 E D C $ 4 F4 R F A student needs to run 3.2 km to get to school. If the student can run 5.5 mi/hr, how many hours will it take them to get to school? 1 ADD FACTOR *( ) % 5 V 18 mi/hr 9 FS T G 0.58 A 10 6 60 km Question 29 of 31 B 0.36 Y min 1.609 5.5 H ANSWER -O 35 & 7 mi 44 F7 0.94 U N hr 00 * 8 J RESET ? 0.6214 3.2 DII FB I M ( 9 K DD F9 O < ) 0 L 4 F10 P - . V : 4 F11 11 + { [ " ? 1.4 11 I 4 F12arrow_forward↑ Na → H H H NaOH, H₂O heat A Version: 1.102.0+ production Problem 35 of 40 Na + H H + S :O: H Undo H Reset I :I: Done Submit Drag To Pan + 8 Tarrow_forward
- Take Test: F23 Virtual Exam 1-2 x 4 ez Launch Meeting - Z... Grammar Help Co... edu/webapps/assessment/take/take.jsp?course_assessment_id=_2600851_1&course_id=2313023_1&content_id= 80124704_1&q LehmanQ- Lehman..... EasyBib®: Free Bibli... Esusu Remaining Time: 1 hour, 02 minutes, 15 seconds. *Question Completion Status: Question 4 R Moving to the next question prevents changes to this answer. New Tab Moving to the next question prevents changes to this answer. 9 How many total atoms are there in one formula unit of Pd3(PO4)4? 5 Q Search 6 Y & 7 U 4+ k 8 X 1 ( + 9 O O P Pearson Sign In O ( + [ DIYarrow_forwardplease answer this questionarrow_forwardArrow pushing mechanismarrow_forward
- calculate the flow rate in ml/hr . order 40meq/l at 10meq/hr in 1000ml in d5w supply 20meq/10mlarrow_forward7. A straight horizontal pipe with a diameter of 1.0 cm and a length of 50 m carries oil with a coefficient of viscosity of 0.12 N-s/m2. At the output of the pipe, the flow rate is 8.6 x 10³ m³/s and the pressure is 1.0 atm. Find the gauge pressure at the pipe input.arrow_forwardIs this mechanism correctarrow_forward
- Calculate the total flow for a 35% venture mask set at 10 liters per minute at the flow meter?arrow_forwardChrome File Edit View History Bookmarks Profiles Tab Window Help A 84% Wed 8:14 T WPAL 101_ 233 _Spring 2022 O NEW BEST Builder Hall 5 Base x ALEKS - David Teague - Learn x X Grades for David Teague: CHM A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZI6tTytly4Fcfu6zOtOf80MM9sfGh3NIP72XeoPmXkrk4zqM9ylm-ZZhvViL. O ☆ D Paused O CHEMICAL REACTIONS David Writing a chemical equation from a description of the reaction 2/5 Aqueous potassium nitrate (KNO3) and solid silver bromide are produced by the reaction of aqueous potassium bromide and aqueous silver nitrate (AgNO,). Write a balanced chemical equation for this reaction.arrow_forwardExplain pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY