
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:-
tab
s lock
introl
<
STARTING AMOUNT
esc
Tap here or pull up for additional resources
option
!
1
L
A
FI
Q
X
N
@
2
W
S
X
H
#
3
80
F3
E
D
C
$
4
F4
R
F
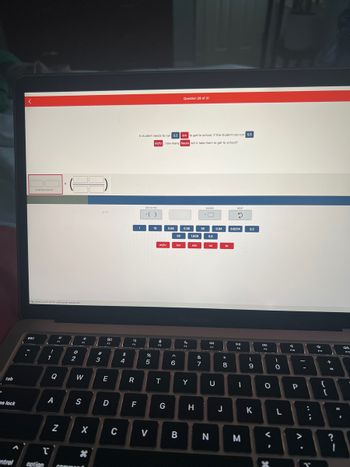
A student needs to run 3.2 km to get to school. If the student can run 5.5
mi/hr, how many hours will it take them to get to school?
1
ADD FACTOR
*( )
%
5
V
18
mi/hr
9
FS
T
G
0.58
A
10
6
60
km
Question 29 of 31
B
0.36
Y
min
1.609 5.5
H
ANSWER
-O
35
&
7
mi
44
F7
0.94
U
N
hr
00 *
8
J
RESET
?
0.6214 3.2
DII
FB
I
M
(
9
K
DD
F9
O
<
)
0
L
4
F10
P
-
. V
:
4
F11
11 +
{
[
"
?
1.4
11
I
4
F12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A runner wants to run 11.0 kmkm . She knows that her running pace is 7.3 mi/h. How many minutes must she run?arrow_forwardA runner wants to run 12.1 km. She knows that her running pace is 7.0 mi/h how many minutes must she run?arrow_forwardThe men's world record (as of 2007) for swimming 1500 m in a long course pool is 14 min 34.56 s. At this rate, how many seconds would it take the men's world record holder to swim 0.250 mi? (1 mi = 1609 m)arrow_forward
- How much money will it cost you to drive 145.0 miles if your car gets 25.70 miles per gallon and gas costs $3.309/gallon?arrow_forwardA blood sample of 2.81 milliliters is collected from a patient to be anaylzed for a platelet count. Human blood should have around 1.04 kg/L platelets. What is the expected mass in grams of platelets in blood samples.arrow_forwardif i start in washington dc , and drive to new york, how many seconds will it takes if i drive 54 miles per hour? the trip is 239 miles.arrow_forward
- A runner wants to run 11.0 kmkm. Her running pace is 8.2 mi/hrmi/hr. How many minutes must she run?arrow_forwardA bottle of liquid medication contains 236mL and costs $5.85. If the usual dose is two tablespoons, how much does each dose cost? (1 tablespoon =15 mL),arrow_forwardYou are renting a car and you wish to travel a distance of 850. miles. If the car has a mileage of 10.1 km/L, how many gallons of gasoline will you need to travel the entire distance?arrow_forward
- [References] You are driving in Canada where the distances are marked in kilometers. The sign says that you are 369 km from Ottawa. You are traveling at a speed of 72.0 mi/h. How long will it take you to reach Ottawa? hoursarrow_forwardA sprinter set a high school record in track and field, running 200.0 mm in 20.9 ss . What is the average speed of the sprinter in kilometers per hour? Where do I start?arrow_forwardHow much money will it cost you to drive 205.0 miles if your car gets 25.70 miles per gallon and gas costs $3.309/gallon?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY