
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!
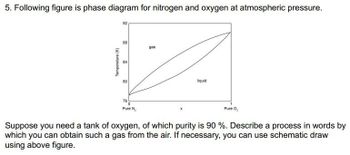
Transcribed Image Text:5. Following figure is phase diagram for nitrogen and oxygen at atmospheric pressure.
Temperature (K)
92
88
84
gas
8
liquid
76
0
Pure N₂
Pure O₂
Suppose you need a tank of oxygen, of which purity is 90 %. Describe a process in words by
which you can obtain such a gas from the air. If necessary, you can use schematic draw
using above figure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- b My Questions | bartleby N MyLab and Mastering O Chem 131 Ch 8 Flashcards | Quiz x Course Home A openvellum.ecollege.com/course.html?courseld=16962209&OpenVellumHMAC=7148a2eb283445d6406a0f326efaf3a4#10001 O My Courses C-C 348 C= C 614 C-H 413 H– Cl 431 C-C1 328 O 304 O -38 O -44 O 38 O 2134 ENG 3:36 PM P Type here to search 68°F 梦 的 US 11/5/2021 近arrow_forward13 ← CO Biblio Manuels-fren.... CH Draw the curved arrows showing a proton transfer reaction, and draw the products of that proton transfer. Do not include the Li+ counterion, and lone pairs are not required in the products. + H-O-H Edit Drawing CMCbx bbwb + C .../1 11:11 PM 2024-02-09 0 AINarrow_forwardOH OH 1.OSO4 2. NaHSO3, H3O OH Select to Edit >arrow_forward
- in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forwardneht_805_Hydrates_1_2_1 (1) (Protected View) - Word (Unlicensed Product) rences Mailings Review View Help Foxit PDF Tell me what you want to do n contain viruses. Unless you need to edit, it's safer toay in Protected View. Enable Editing rour Office product is inactive. To use for free, sign in and use the Web version. Activate Use free at Office.com Part 3: Reversibility of hydration 1. Transfer a small amount of solid copper (II) sulfate pentahydrate, CUSO4-5H;O, that just fills the bottom of a clean, dry 150-mm (medium size) test tube. 2. Using a test tube holder, grasp the test tube containing the hydrate and heat over a Bunsen burner flame while holding the test tube at a 45° angle. 3. While heating, closely observe the solid and the inside wall of the test tube. Record your observations. 4. When the solid residue seems to be completely dehydrated, allow the test tube to cool completely. Then, add a few drops of laboratory water to Record your observations. solid in the test tube.…arrow_forwardChrome File Edit View History Bookmarks People Tab Window Help Bb LX Bb Bb 台 h + A labflow.com/app/course/362/report/7684#tab=REPORT : Apps B5 Blackboard Mail - Ava Schied... OUAConnect e Biology Syllabus >> REPORT SUMMARY Water Cyclohexane tF KMN04 Soluble Insoluble 12 Insoluble Soluble Sucrose Soldole Insoluble Vegetable oil Insoluble Soluble Electrolytes Lightbulb observation for 0.1 M NaCl Bright glow Lightbulb observation for 0.1 M sucrose No light Lightbulb observation for 0.1 M HCI Bright glow Lightbulb observation for 0.1 M acetic acid (HC2H302) Dim glow Lightbulb observation for 0.1 M NaOH Bright glow Lightbulb observation for 0.1 M NH,0H Dim glow Lightbulb observation for 0.1 M ethanol (C2H5OH) No light Concentration Mass of empty evaporating dish (g) 27.761 Volume of NaCl solution (mL) 9.9 Mass of evaporating dish and NaCl solution (g) 38.770 Mass of evaporating dish and dry NaCl (g) 29.450 OCT 3 T Warrow_forward
- Experiment 605_Hydrates_1_2_1 (1) (Protected View) - Word (Unlicensed Product) ces Mailings Review View Help Foxit PDF Tell me what you want to do contain viruses. Unless you need to edit, it's safer to stay in Protected View. Enable Editing ir Office product is inactive. To use for free, sign in and use the Web version. Post-lab Questions Activate Use free at Office.cc 1. Calculate the mass percent of water for the hydrate, LINO,-3H;O. 2. What will be the probable effect if you kept the crucible completely covered during the entire heating and cooling processes? Would your calculated percent water in the hydrate be high, low, or unaffected? Explain. 3. If 2.752 g sample of Ca(NO:); XH;O is heated to constant mass, the residue weighs 1.941 g. Determine the value of x and the formula of the hydrate.arrow_forwardW AutoSave On homewrok - Compatibility Mode - Saved - O Search (Alt+Q) raghav grover RG File Home Insert Design Layout References Mailings Review View Help O Comments A Share Draw - A A Aa v A O Find Times New Roman v 12 Normal Body Text List Paragraph No Spacing E Replace Paste В I U v ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard s Font Paragraph Styles Editing Voice Editor Reuse Files 14. Outline a synthetic route for converting A into B. This will require several reaction steps. Show the reactant, reagents and product for each step. You do not need to show the mechanisms. OH B A Page 15 of 19 1243 words English (United States) Accessibility: Unavailable D Focus ENG 3:15 PM O Type here to search 6°F US 2021-12-16 后arrow_forwardStearic acid: Description of bromine (red-orange) color persistancearrow_forward
- OnCourse Connect Assessment: Chemistry x Copy of spread_of_islar x = The Spread of Islam -G x M Inbox (904) - aarojame A Classes boncourseconnect.com/assessment/1651879/5287e2a3-0d0b-e2c0-c15c-3e68a37149b4 D TPSS Bookmarks CHEMISTRY BENCHMARK TEST 01 CHEMISTRY I 12-7 (AARON JAMES, ID: 12390724) A student dissolved 15 grams of pure acetic acid (CH,COOH) in enough water to make 100. milliliters of solution. Given the atomic masses provided on the periodic table, what is the molarity (M) of the acetic acid solution? a 0.025 molar D0.40 molar © 2.5 molar O 4 molar A B B 1 2 4 5 6 7 8 9 10 3. O Save/Exit 4 sn TESSarrow_forwardWe want to inform you that your performance as a tutor is being evaluated and will be reported to the administration. Additionally, there will be an online ranking system that not only assesses the overall performance of Bartleby as a website but also ranks individual tutors based on their question-and-answer accurate contributions. This ranking will be shared with management. Please ensure that your responses are of the highest quality, as multiple requests may be directed to the same person for assessment.arrow_forwardO Homework in Chem101 - due Su X My Questions | bartleby ( Periodic Table – Royal Society of how to take a screenshot on an + A learn.maricopa.edu/courses/1132256/modules/items/19018052 CHM151 17003 > Modules > Weeks 8 & 9 - Chapter 7 > Homework in Chem101 - due Sunday night CG 2020 FALL CRED Question 38 of Submit Account Home Complete the balanced neutralization equation for the reaction below: Announcements Dashboa |Modules HCIO:(aq) + NaOH(aq) – rd Concourse Syllabus Courses Grades O3+ D4+ Cisco Webex Groups 1 2 3 4 7 8 9. Tutoring/Learning Center Calendar Os Do Inbox (1) (g) (aq) History Na CI H. Help • Previous Next » Library 1:03 PM O Type here to search 10/18/2020 1L + 近arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY