
Principles of Modern Chemistry
8th Edition
ISBN: 9781305079113
Author: David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Can someone explain this?
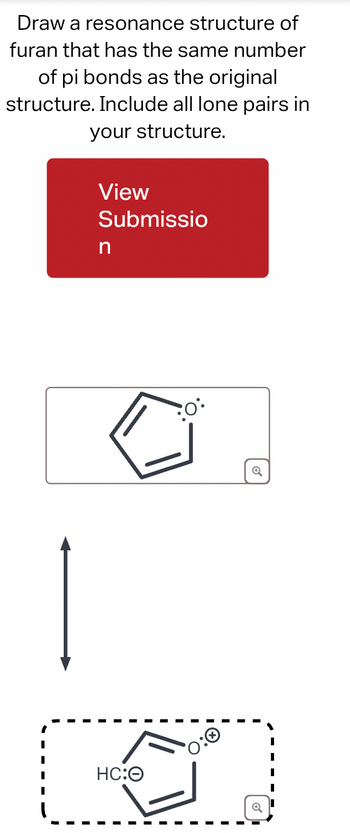
Transcribed Image Text:Draw a resonance structure of
furan that has the same number
of pi bonds as the original
structure. Include all lone pairs in
your structure.
View
Submissio
n
HC:0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Carbon monoxide (CO) is an example of an overall neutral molecule (netcharge=0) that hasnon-zero formal charges. Draw a Lewis structure of carbon monoxide (CO).arrow_forwardIn the Lewis structure for chloromethane, the chlorine atom is sharing _____ electron pair and “owns” _____ of those electrons. Also, the chlorine atom possesses two electrons from each of _____ unshared pairs. The total number of electrons that belong to chlorine is 7 . Chlorine is a Group ____ element. The formal charge on chlorine in chloromethane is ____.arrow_forwardPlease draw all Lewis dot structures for each species below. Ifthere is more than one, please circle which you’d argue is thebest structure. Also denote the formal charges for each atom.arrow_forward
- Please complete the chart belowarrow_forwardWhich of these best descibe formal charge? Select all that apply. The difference between the number of electrons around an atom in the free state and the number of electrons assigned to the atom in the Lewis structure an atom in a chemical compound. O The formal charge of each atom is calculated by subtracting the number of valence electrons in the neutral atomfrom the number of electrons assigned to the atom. O Can be used to help determine the most reasonable distribution of electrons in a molecule or ion. O The charge that an atom in a molecule or ion would have if all atoms had the same electronegativity.arrow_forwardNhy is resonance structure A and resonance structure B not the same structure? If you rotate the molecule like a spicket handle (l. e. clockwise with the C at the center and the R group remaining in place as you look down the C - R bond) wouldn't you get the same thing? Why are these not equivalent structures? Resonance structure A t Resonance structure B Actual structurearrow_forward
- central atom underlined: NH3O ICl3 XeO3 C4H4 O.R.-Lewis structure: Remember to always: • Show charge, if present. • Draw at least one more equivalent resonance structure (with «) if present, to describe delocalized covalent bonding. count all lone pairs in LS: polar: Y/N?arrow_forward2. Draw the Lewis structure of AlIF, 3 Indicate ubether the hond that occurs betwen each pair ofarrow_forwardDescribe the difference between a full Lewis structure and bond-line notation. What changes? Why is it easier to write?Dontarrow_forward
- Which of the following pairs are NOT resonance structures? + H₂C-0-N=0: and H₂C-O=N-0: :0=c=0: =Ö: and :Ö= H₂C-0-N=0: and H₂C- Each of these pairs represents resonance structures. O None of these pairs represents resonance structures.arrow_forwardWhich of these resonance structures contributes most to the overall structure of the molecule? 0=S=0 :ö-s- =s- ö-s=ö :0-s= :0::ö: :ö: :0:arrow_forwardProvide ALL reasonable resonance structures for each molecular structure below.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co