
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:<
8:02
Question 11 of 25
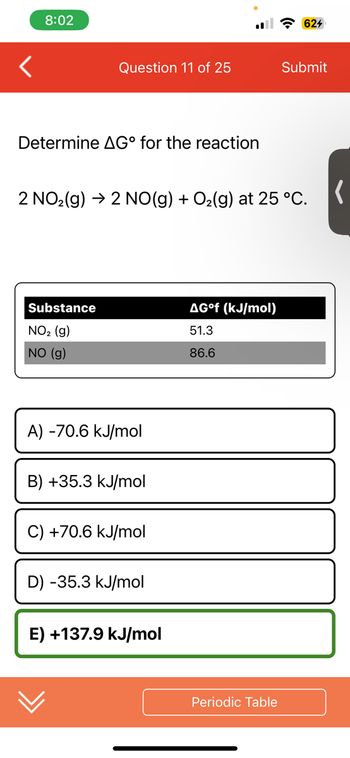
Determine AG° for the reaction
Substance
NO₂ (g)
NO (g)
2 NO₂(g) → 2 NO(g) + O₂(g) at 25°C.
A) -70.6 kJ/mol
B) +35.3 kJ/mol
+70.6 kJ/mol
.624
D) -35.3 kJ/mol
E) +137.9 kJ/mol
AG°f (kJ/mol)
51.3
86.6
Submit
Periodic Table
Expert Solution
arrow_forward
Step 1 Formula
∆G° = Sum of Gibbs free energy of formation of products - Sum of Gibbs free energy of formation of reactants
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction. 2A(g)+B(g) = 4C(g) H=-107 kJ/molUnder which reaction conditions would you expect to have the greatest equilibrium yield of C(g)?arrow_forwardConsider the following reaction at 298 K. 2 so2(g) + 02(9) → 2 so3(g) An equilibrium mixture contains 0,(g) and So, (g) at partial pressures of 0.49 atm and 2.7 atm, respectively. Using data from the Thermodynamic Data table, determine the equilibrium partial pressure of So, in the mixture. 7.2e12 X atmarrow_forwardAt what temperature, in °C, is a certain reaction at equilibrium if AH = +83.8 kJ/mol and AS = +170.2 J/mol · K?arrow_forward
- Ag(+aq) + Cl-(aq) AgCl(s) ∆H = +112 kJIf ions are colorless, and AgCl is whiteWill the reaction appear “colorless” or “white” if:a) Some of the AgCl (s) is removed?b) The pressure is decreased?c) The temperature is raised?arrow_forwardFor a particular reaction at 180.9 °C, AG = 527.78 kJ/mol, and AS = 953.96 J/(mol · K). Calculate AG for this reaction at -3.7 °C. AG = kJ/molarrow_forwardBelow what temperature does the following reaction become nonspontaneous? 2 HNO3(aq) + NO(g) → 3 NO2(g) + H2O(l) ΔH = +136.5 kJ; ΔS = +287.5 J/K A) 39.2 K B) 151 K C) 475 K D) 501 Karrow_forward
- Carbon monoxide reacts with water according to the following equilibrium reaction: CO (g) H2O (g) CO2 (g) + H2 (g) For this reaction, K = 0.810 at a temperature of 1000.°C. Calculate the value of AG for this reaction at 1000.ºC. %3Darrow_forwardConsider the reaction: PbCl2(s) → Pb2*(aq) + 2 Cl (aq) AH° = 23.30 kJ/mol and AS° = -12.5 J/K-mol a) When solid PbCl2 is dissolved in water at 25°C, what are the concentrations of Pb2* and Cl at equilibrium? (Hint: Do your ICE chart and Law of Mass Action.) Concentration of Pb2+: mol/L and Concentration of Cl = mol/L (Do not use superscripts, subscripts, or carets. Write 1.2 x 10-3 as 1.2x10-3 or 1.2e-3.) b) At 25°C, This reaction is (reactant or product) favored. Justify your answer with the appropriate calculation on the separate sheet of paper. c) The Gibbs free energy at 25°C, when both the concentrations of the lead ion and chloride ion are 1.2 x 10-³M is kJ/mol. Show your calculations on the separate sheet of paper.arrow_forward17. The thermochemical equation for the reaction of F2 and water is as follows. AH = -270 kJ F2(g) + 2 H2O (liq)=HF (aq) + O2 (g) Given a system that is initially at equilibrium, which of the following actions cause the reaction to proceed to the right? a) adding O2(g) b) decreasing the temperature c) adding a catalyst d) decreasing the volume of the reaction chamber e) none of the abovearrow_forward
- The equilibrium constant, Kp, for the following reaction is 1.57 at 600 K.CO(g) + Cl2(g) COCl2(g)If ΔH° for this reaction is -108 kJ, what is the value of Kp at 699 K?Kp =arrow_forwardCalculate the temperature at which the reaction below will be at equilibrium, then explain how this temperature can affect the spontaneity, meaning how using higher or lower than this temp can affect the value of delta G. Given. Br2(l) → Br2 (g), ΔH = 31.0 kj/mol ΔS = 92.9 j/molarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY