
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
4
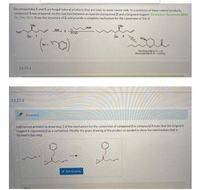
Transcribed Image Text:Decytospolides A and Bare fungal natural products that are toxic to some cancer cells, In a synthesis of these natural products.
compound 3 was prepared via the reaction between an epoxide (compound 2) and a Grignard reagent (Tetrahedron: Asymmetry 2015.
26, 296-303). Draw the structure of 2, and provide a complete mechanism for the conversion of 1 to 3.
OBn
OBn
NaH
TeO
2) H0
Bn
Decytonpolide A R-H)
Decytospolide R- COCH)
13.25 a
13,25 d
Incorrect.
Add curved arrow(s) to draw step 1 of the mechanism for the conversion of compound 2 to compound 3 (note that the Grignard
reagent is represented as a carbanion). Modify the given drawing of the product as needed to show the intermediate that is
formed in this step.
Edit Drawing
Hint
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q5. As part of the aspirin synthesis lab, the orgo students also had to perform the following calculation to demonstrate their knowledge. Are you able to help them work this out?Saponification is a process in which soap is produced from the chemical reaction between animal fat (triglycerides) and a strong base such as NaOH. An example of such a balanced chemical reaction is shown here:C57H110O6 + 3NaOH à C3H5(OH)3 + 3C18H35O2NaIf, during the saponification reaction, 228.5 g of C57H110O6 is mixed with 211.7 g of NaOH and 180 g of soap is produced: a. Calculate the theoretical yield of soap (in grams), C18H35O2Na, and indicate which species is the limiting reactant. Provide your answer to 2 decimal places. b. Calculate the percent yield for this reaction. Provide your answer to 1 decimal place. Show ALL steps and equations involved in your calculations. Remember to label all steps clearly and use appropriate unitsarrow_forward24 25 26 A chemistry student weighs out 0.0202 g of acrylic acid (HCH,CHCO,) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1800M NAOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Round your answer to 3 significant digits. mL x10 Continue Submit Assignment 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility C 80 000 000 F1 F2 DII DD F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 @ 2$4 % &arrow_forwardThe O'Keefe's have 50 gallons of cucumbers for pickling. They have supplies of potassium hydroxide, sulfuric acid and ethanol on hand. The 1.5M KOH solution costs $5 per liter, 9 M H2SO4 solution costs $7.50 per liter, and ethanol costs $25 per liter. A 50 g jar of alum from the grocery store costs $8.59. Do you think it’s worth the time and trouble for Richard and Diane to make their own alum from discarded aluminum cans? Explain your answer showing all calculationsarrow_forward
- (4) Ag20, NH4OH H.arrow_forward2 2 X. Fo fff O-S-CH3 01S1O 0-S-CH3 F OTS CH3NH DMSO Br DMF Br EtOH CH3NH DMSO Yor + Br - Br X -S-CH3 -S-CH3 NHCH3 + OTS OP NHCH3 + Farrow_forward2.) Five standardized dilutions of aqueous lead(11) chromate solution were prepared by adding a volume of 0.0010 M PbCrO4(ag) from a buret and then diluting the solution with deionized water to a total volume of 100.0 mL. Use the provided information to determine [CrO42] in each. Hint: enter the decimal before the scientific notation. Mixture Initial Buret Reading (mL) Final Buret Reading (mL) [Cro,?] (M) 3 2.00 7.10 ? x 105arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY