
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
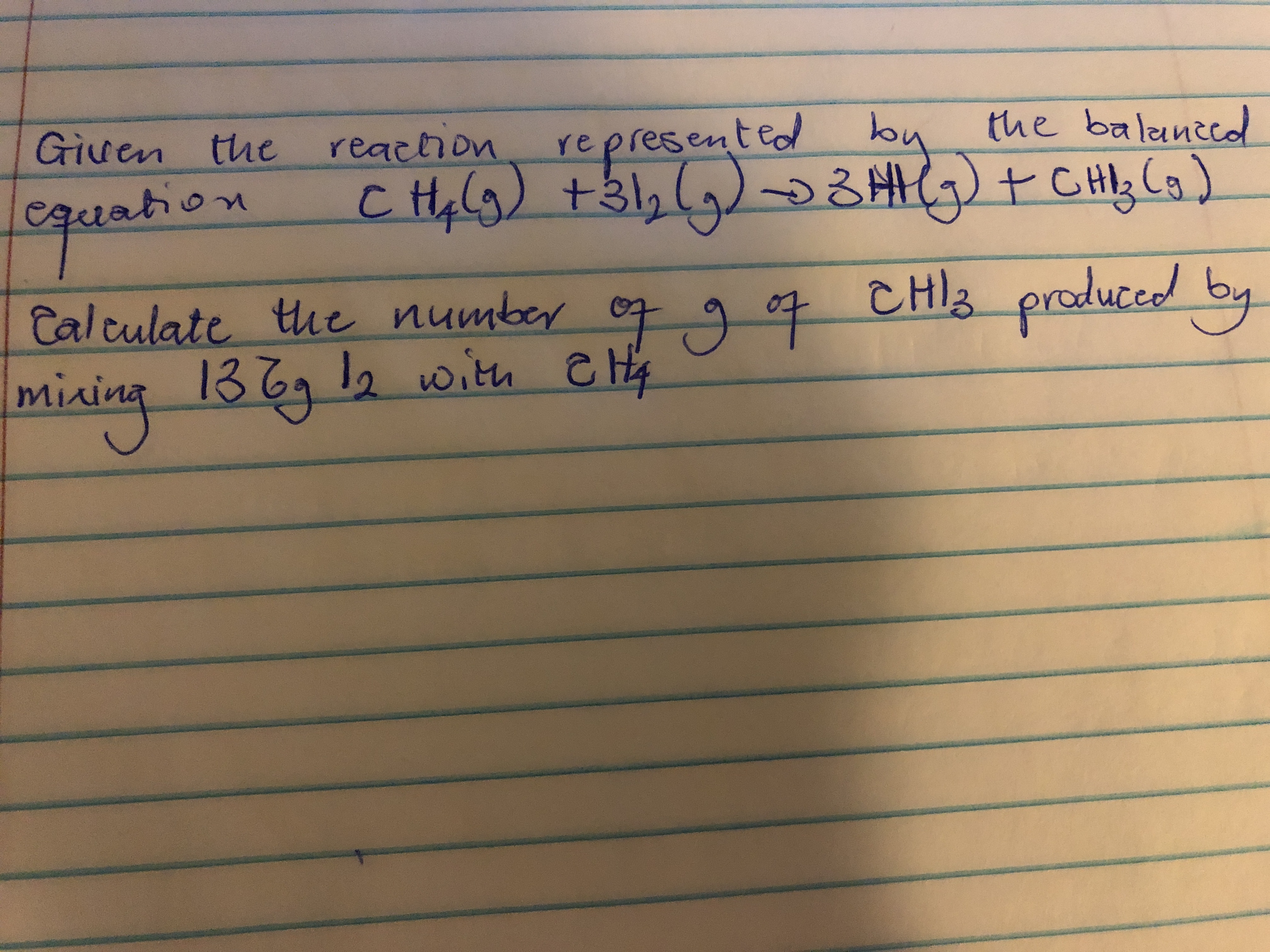
Transcribed Image Text:Given the reachion
represented by
C Hy)+32 (5)る州)+ CHy Cs)
the balanced
equation
Calculate te
number o 907
CHIZ
produccd by
mixina
mining 1369 l2 with e Hy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Similar questions
- D21 Quizzes - Spring 2023 × | с < + Your Chemistry answe × b Answered: Balance th × app.101edu.co 1 Balance the following chemical equation (if necessary): BF (s) + Li₂SO₂ (s) → LiF(s) + B₂(SO₂),(s) + 04- Reset 2 O B₂(SO3)3 0₂ 0 ♡ 3 3 Aktiv Chemistry ) Time's Up! 4 □ 5 Li₂SO₂ л n = O) 04 05 06 6 + Ch 12-14, 18 Public sp X 2+ 7 07 BF (s) (1) 3 ल ³+ 4+ 8 9 ☐8 (g) D2L Quiz 10-12 - Spring 20 X • x H₂O LiF 0 口。 (aq) Delete b Success Confirmation x | + Update: Submitarrow_forward(a) Iz(s) + 5 Cu²* (aq) + 6 H2O(1) (b) Hg²*(aq) + 21 (aq) (c) H2SO3(aq) + 2 Mn(s) + 4 H*(aq) → S(s) + 2 Mn²* (aq) + 3 H,O(I) 2 10;"(aq) + 5 Cu(s) + 12 H*(aq) - Hg(1) + I2(s) - -arrow_forwardBalance the following chemical equation and determine the lowest whole numbered coefficient for H2O Al4C3(s)+H2O(l)——>CH4(g)+Al(OH)3(s)arrow_forward
- 37R37S nL of OilooM ealetum chloride reacts complalely with aques lyer nitrate iuhat s the 143.32 al mol) O.100M eaet (१०७.९ Co९० ) mass of Aa C! precipitale? Caclalag)+2arrow_forwardgiven the following equation 2 Co2O3(s) + 3 C(s) --> 4 Co(s) + 3 CO2(g) indicate the coefficient for elemental carbon 6 4 1 3 or 2arrow_forward7:25 ◄ Search 2- 3c₂²- 1 + Write a balanced chemical equation based on the following description: solid barium carbonate decomposes into solid barium oxide and carbon dioxide gas when heated BaCO3 (s) (s) Be O Question 5 of 20 Reset 2 3 4 5 6 7 8 B (1) Ox Ba Cb (g) C 个 Br Tap here or pull up for additional resources 5G 85 Ca Submit 9 11 (aq) O Cr • x H₂Oarrow_forward
- FeSO4 + Potassium phosphate à Iron (II) phosphate + K2SO4 State the Molar Ratio of the reaction.arrow_forwardter answ Aqueo us sulfurous acid (H2S03) was made by dissolving 0.200 L of sulfur dioxide gas at 19°C and 745 mm Hg in water to yield 500.0 mL of solutio n. The acid solution required 17.6 mL of so dium hydroxide solution to reach the titration end point. What was the molarity of the sodium hy droxide solution? M NaOH IID $4 7. COarrow_forwardHow many moles of Ca(OH) 2 are required to completely react with 300.0 mL HCl solution? (HCl content of the HCl solution is 18.00% HCI by mass and density of the HCl solution is 1.200 g/mL) Reaction: Ca(OH) » (s)+ 2HC1 (aq) CaCl: (aq) + 2H:O (1) Lütfen birini seçin: O a. 113.5 mol O b. 0.112 mol O c. 0.889 mol O d. 2.42 mol O e. 1184 molarrow_forward
- i need the answer quicklyarrow_forward1-1. The raw water supply for a community contains 18 mg/L total particulate matter. It is to be treated by addition of 60 mg alum (Al2(SO4)3-14H;O) per liter of water treated. Essentially, all the added alum precipi- tates represented by the following reaction: Al2(SO4)3 · 14H20 → 2 Al(OH);(s) + 3So,- + 6H* + 8H2O For a total flow of 7500 m/d, compute the daily alum requirement, the total concentration of suspended sol- ids in the water following alum addition, and the daily load of particulate solids requiring disposal (including both those initially present and those formed during treatment).arrow_forward(a) Iz(s) + 5 Cu²* (aq) + 6 H;O(I) –→ 2 103"(aq) + 5 Cu(s) + 12 H*(aq) (b) Hg²*(aq) + 21 (aq) → Hg(1) + Iz(s) (c) H2SO3(aq) + 2 Mn(s) + 4 H¯ (aq) · S(s) + 2 Mn²*(aq) + 3 H2O(I)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY