
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
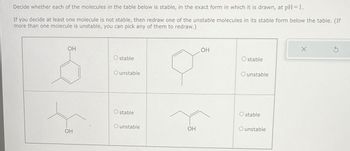
Transcribed Image Text:Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 1.
If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If
more than one molecule is unstable, you can pick any of them to redraw.)
OH
O stable
O unstable
OH
X
5
O stable
O unstable
O stable
O stable
O unstable
OH
O unstable
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Starting from the following alkene, which products will be obtained upon the action by the following reagents? Match the letter for the product, if found in the list, to each reagent. A НО. E Br reagent НО. B Br F t C Br G OH Br D H Brarrow_forwardUsing the table of bond dissociation energies, the AH for the following reaction is CH4 + 20 2→ CO 2 + 2 H2O Bond D (kJ/mol) C-H 413 O=O 495 =0 799 463 -52 154 -1246 684 -808 kJ.arrow_forwardОН ОН -OH ОН ??? ??? ??? ??? ОН ОН Br ОНarrow_forward
- Consider the acid-base reaction between iodoethane (CH3CH2I) and hydroxide (-OH). CH3CH2I + OH- --> CH3CH2OH + l- Use the table of bond energies to estimate the change in enthalpy (∆H in kJ/mol) for this reaction. Think about the bonds that are broken and that are formed. This reaction is endothermic, exothermic or energy neutral Using your calculation, what can you say about the strengths of the bonds? -The bonds before the reaction are stronger than the bonds after the reaction. -The bonds after the reaction are stronger than the bonds before the reaction. or -The bonds before and after the reaction are equivalent in strength.arrow_forward8) Label the energy states in the diagram below (options are 1s4d, 1s3d, 1s3s). You will need to think about which energy gap is the largest, which energy gap is the smallest. This is an idealized energy diagram (shape is simplified, focus on distance from nucleus) 1s2parrow_forwardCO₂H -OH -OH heat 1. SOCI 2, A 2. NaO,CCH2CH3 ?arrow_forward
- Compound ∆Hf (kJ/mole) ∆S (J/mole K) C2H4 52.4 219.3 C2H6 –84.68 229.2 SiO2 –910.7 41.5 HF –273.3 173.8 SiF4 –1615.0 282.8 NH3 –45.9 192.9 O2 1.88 2.43 NO 91.3 210.8 H2O(g) –241.8 188.8 For the following reactions, using the provided ∆H and ∆S data, determine whether each one would be spontaneous or nonspontaneous at 25°C. C2H4(g) + H2(g) --------à C2H6(g) SiO2 + 4 HF(g) -------------> SiF4 (g) + 2 H2O(g) 4 NH3(g) + 5 O (g) ---------> 4 NO(g) + 6 H2O(g)arrow_forwardCompound ∆Hf (kJ/mole) ∆S (J/mole K) C2H4 52.4 219.3 C2H6 –84.68 229.2 SiO2 –910.7 41.5 HF –273.3 173.8 SiF4 –1615.0 282.8 NH3 –45.9 192.9 O2 1.88 2.43 NO 91.3 210.8 H2O(g) –241.8 188.8 4 NH3 + 5 O2 -> 4 NO + 6 H2Oarrow_forwardEnergy in the reaction center comes from bond energy. red light. absorbed photons. green light. potential energy. перов d OO d a b Uarrow_forward
- Which of these three is most stable? Explain (justify)arrow_forward7. Estimate the enthalpy of reaction (in kJ/mol) for the combustion of methane. Be sure to show a lewis structure for each molecule. CH, (g) + 20, (g) ·CO, (g) + H,O (g) Bond Bond Enthalpy (kJ/mol) C-O 351 C=0 799 CEO 1070 O-O 142 O=0 498 C-H 414 O-H 460arrow_forwardparts b and c pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning