
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
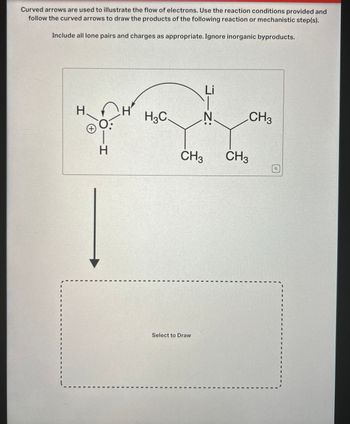
Transcribed Image Text:Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and
follow the curved arrows to draw the products of the following reaction or mechanistic step(s).
Include all lone pairs and charges as appropriate. Ignore inorganic byproducts.
H
(+)
H-
H3C
N
Li
J-Z:
CH3
CH3
CH3
Select to Draw
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. MgBr CO2 Select to Drawarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts. :OH Draw Product -HCI Draw Tetrahedral Intermediatearrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Then draw the organic product of the reaction. Include all lone pairs in the structures. Ignore inorganic byproducts and + counterions. Drawing Arrows NaNH2 Select to Draw Product H H I I O:Z: + S :O: H Undo H Na+ EP Resetarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the products of the following reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Į Br: :Brarrow_forwardCurved arrows are used to illustrate the flow of electrons. Follow the arrows and draw the intermediate and product in this reaction. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts. I I I I H N. H HCI Drawing H o I I I I Iarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions and follow the curved arrows and draw the intermediate and product in this reaction sequence or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore any inorganic byproducts.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts. N. CH3I > H&C aarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. :0: :0: CH₂N2 NEN HH N. Q LAVEN Probl Atom and Draw orarrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows and draw the anion formed in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore any inorganic byproducts. H N: H Qarrow_forward(please don't use hend raiting)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning