
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
![Determine the initial [SCN1] in beakers 1-6. Refer to the Data Table for Part I
for all volumes used. Assume for now that these [SCN1] values are equal to
the equilibrium [FeSCN2] in beakers 1-6 because of the large excess of Fe*³
used.
Prepare a graph of Absorbance vs [FeSCN+2]. Remember that according to the
Beer-Lambert Law, this graph should be a straight line. Scale your graph so
that the [FeSCN2] can be estimated to the nearest 0.01 x 104 M and so that
your absorbance values can be estimated to the nearest 0.001.](https://content.bartleby.com/qna-images/question/97ff73dd-3f4a-48f5-beb4-1856d5a5bcd8/7a32b577-87fa-41ef-85b5-4f5d1ce89fd6/ettrn1_thumbnail.jpeg)
Transcribed Image Text:Determine the initial [SCN1] in beakers 1-6. Refer to the Data Table for Part I
for all volumes used. Assume for now that these [SCN1] values are equal to
the equilibrium [FeSCN2] in beakers 1-6 because of the large excess of Fe*³
used.
Prepare a graph of Absorbance vs [FeSCN+2]. Remember that according to the
Beer-Lambert Law, this graph should be a straight line. Scale your graph so
that the [FeSCN2] can be estimated to the nearest 0.01 x 104 M and so that
your absorbance values can be estimated to the nearest 0.001.
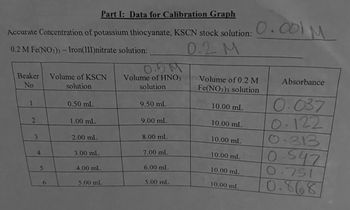
Transcribed Image Text:Beaker Volume of KSCN
No
solution
1
0.50 mL
9.50 mL
Part I: Data for Calibration Graph
Accurate Concentration of potassium thiocyanate, KSCN stock solution: 0.001 M
0.2 M Fe(NO3)3- Iron(III)nitrate solution:
0.5M
Volume of HNO3
solution
0.2 M
Volume of 0.2 M
Fe(NO3)3 solution
10.00 mL
Absorbance
0.037
2
1.00 mL
9.00 mL
10.00 mL
3
2.00 mL
8.00 mL
10.00 mL
4
3.00 mL
7.00 mL
10.00 mL
0.122
0.313
0.547
5
4.00 mL
6.00 mL
10.00 mL
6
5.00 mL
5.00 mL
10.00 mL
0.791
0.868
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Patients undergoing an upper gastrointestinal tract laboratory test are typically given an X-ray contrast agent that aids with the radiologic imaging of the anatomy. One such contrast agent is sodium diatrizoate, a nonvolatile water-soluble compound. A 0.378-m solution is prepared by dissolving 38.4 g sodium diatrizoate (NaDTZ) in.l.60 102 mL water at 3 1.2C (the density of water at 31.2C is 0.995 g/cm3). What is the molar mass of sodium diatrizoate? What is the vapor pressure of this solution if the vapor pressure of pure water at 31.2C is 34.1 torr?arrow_forwardA solution is made by dissolving 0.455 g of PbBr2 in 100 g of H2O at 50C. Based on the data in Table 8-1, should this solution be characterized as a. saturated or unsaturated b. dilute or concentratedarrow_forwardA solution was prepared by dissolving 367 mg of K3Fe(CN)6 (329.2 g/mol) in sufficient water to give 750.0 mL. Calculate (a) the molar analytical concentration and Normality of K3Fe(CN)6. (b) the molar concentration of K+. (c) the molar concentration of Fe(CN)63-. (d) the weight/volume percentage of K3Fe(CN)6.arrow_forward
- What volume in mL of 4.35 M HCI solution is needed to react with 18.5g of Ca(0H)2arrow_forwardA. 1.4639 g sample of limestone was analyzed for Fe, Ca and Mg. The iron was determined as Fe2O3, yielding 0.0357 g Calcium was isolated as CaSO4, yileding a precipitate of 1.4058 g and Mg was isolated as 0. 0627 g of MgP2O7. Report the amount of Febas %w/w Fe2O3, Ca as %w/w CaO and Mg in the limestone sample as % w/w MgOarrow_forwardAns. 17.15 mg. 54) A 1.000-g sample of a mixture which contains only NaCl and KCl gare a precipitate of AgCl which weighed 2.000 g. What are the percentages of Na and K in the mixture? ins 10 g Ans. 44.77% K; 5.75% Na. 1:J 55. I- can be sernarated from othorarrow_forward
- e * OWLV2 | Onlin x * MindTap - Cen x C The SolubilityX b Answered: Ma G is Ags insolubl genow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take [References] The solubility of magnesium fluoride, MgF2, in water is 1.5 x 10-2 g/L. What is the solubility (in grams per liter) of magnesium fluoride in 0.39 M of sodium fluoride, NaF? Solubility = g/L Submit Answer Try Another Version 3 item attempts remaining C OWLV2 | Online tea.. Homo Insertarrow_forwardAgency TEXAS UNITED REALTY Real Estate 75 g u cup increases by 2.44 70 V the heat change per mole of Cich dissolved in water Assame that all the heat is absorbed ed E. D 1 15) When aqueous solutions of Pb(NO3)2 and NaCl are mixed, PbCl2 precipitates. The balanced net ionic equation is: O to slom to slom I asdW (SC flot sdi of gaibros (2)OsH bas (2)5¹1 mot Pb2+ (aq) + 2C1- (aq) → PbCl2 (s) prisupe Pb2+ (aq) + 2NO3- (aq) → Pb(NO3)2 (s) H Pb2+ (aq) + 2NO3- (aq) -> Pb(NO3)2 (aq) Pb(NO3)2 (aq) + 2NaCl (aq) → PbCl2 (aq) + 2NaNO3 (s) laubonarrow_forward- Calculate the concerntration of NaOH in the solution provided.arrow_forward
- slove Part BFor the same procedure described in the chemical equilibrium lab handout for determining K, 27.0 mL of organic solution was added to 65.0 mL of KI aqueous solution at 317.1 K. Both the aqueous and organic solutions were prepared at 298.15 K with the apparent concentration of 0.0796 M for the I-(aq) and 0.0065 for the I2(org) solutions, respectively. After mixing these immiscible solutions, the final concentration of I2 in the organic layer was determined to be 0.0006 M through UV-Vis spectroscopy. In a separate experiment, the partition coefficient was found to be k = 0.01 at 317.1 K. a) Determine the approximate equilibrium constant, K, without making any temperature correction, for the reaction: I2(aq) + I-(aq) ⇌ I3-(aq) at 317.1 K. K = 5294.4 b) Now, make the temperature correction for volume expansion in the calculation of K assuming the solvent is Cyclohexane. What is the percentage error for using the non-corrected K rather than the corrected K?arrow_forwarda) What mass of Fe(NH4)2(SO4)2•2H2O(s) is required to prepare a 500 mL solution containing 100 ppm (m:v) in Fe? b) Using this stock solution, what aliquot must be used to prepare calibration solutions, 100 mL volume, of the following concentrations: 0.100 ppm, 0.500 ppm, 2.00 ppm, 4.00 ppm, and 7.00 ppm.arrow_forwardGg.170.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

