
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
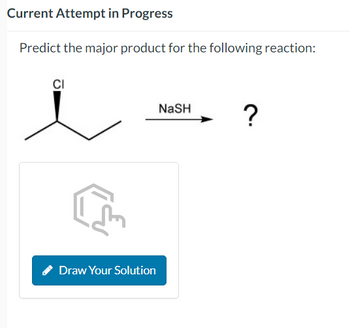
Transcribed Image Text:Current Attempt in Progress
Predict the major product for the following reaction:
CI
Draw Your Solution
NASH
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- The key step in a reported laboratory synthesis of sativene, a hydrocarbon isolated from the mold Helminthosporium sativum, involves the following base treatment of a keto tosylate. What kind of reaction is occurring? How would you complete the synthesis?arrow_forwardIn each reaction box, place the best reagent and conditions from the list provided. 1) Br 2) 3) 4) DEET (the active ingredient in over the counter insect repellant) 5) Answer Bank Mg, Et,0 HN(CH, CH, ), (1 equiv.) H,O* CH,COOH CH,CH,NH, CH,CH, Br NH(CH,CH,),(2 cquiv.) НСООСH, H,CO НСООН CH, CH, OH NaCN NaNH, Br,, FeBr, SOCI, CO,arrow_forwardPlease don't provide handwritten solution ..arrow_forward
- 5. knat reagento are necessary to perfom the following reaction ? Br Br A. C Bry (iequiv), NAOH D. None of the abouldsarrow_forwardQuestion 15 of 30 > O Macmillan Learning In each reaction box, place the best reagent and conditions from the given list. H₂C H3C OH TsCl, pyridine CH3 NaBH4 H₂O₂, NaOH, H₂O 1) 2) 3) 4) (CH3)3 CO™ INDI Answer Bank H₂SO4, conc. CH,O CH3COO H₂C NaOH BH3, THF н' H₂O+ OH CH3arrow_forwardGive correct detailed Solution with explanation needed..don't give Handwritten answerarrow_forward
- What is/are the reagent(s) and conditions for the following reaction? 0: Problem viewing the image, Click Preview Here O NaBH, EtOH O 1. LIAIH4 2. H30¹ H O Zn(Hg), HCI, H₂O O N₂H4, KOH, heat H tx H Harrow_forwardProvide the major product for the reactionarrow_forwardPredict major product Do not give handwriting solution.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning