
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
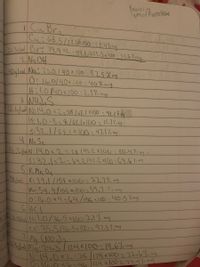
Transcribed Image Text:Balavei
TyAsoffecochions
Cu Br.
Cui 63.5/22381I00 -2R.4%mg
23a aBr: 19.9 *2-159.8/223.3xlo0 - 71.6%mg
2 Na OH
40alud Na: 25.0/40x100:57.5% mg
0.16.0/40x160:40% mg
Hi1.0/40x100:2.5%. mg
3 NH)S
(4:1,0.8=8/68lx(00-11.7%,ng
5:32.1/68.111I00:47.1% ng
4 No Sa
2.2gl4 N:14.0x2-28192.2 X1U : 30.4%i mg v
5:32.1x2=64.2142.2 x1C0 =69.6.1.ag
5K MA O4
58 lul Ki 39,1/158 x100-27.7%, mg
Mn:54,9/156x160=34.7%, my
0:16.0x4-64/158160-40.5 hmng
6HCI
SGal Hil.0/36.5x(60-2.7% mg
C1:35.5/36.5x100=97.3%. my
Mg (NO)2
Ma 24.3 /124K100-19.6lo
N:14.0x2-24/124x100=22.6%. mg
mg
Expert Solution
arrow_forward
Step 1
Note: As per our guidelines, we are supposed to answer only the first three questions (i.e. 1 to 3 ). Kindly repost other questions separately.
Standard data:
- The molar mass of CuBr2 = 223.3 g/mol
- The molar mass of NaOH = 40 g/mol
- The molar mass of (NH4)2S = 68.1 g/mol
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Assignment/takeCovalentActivity.do?locator=assignment-take Submit Answer $ R F www An aqueous solution of ammonium sulfide, (NH4)2S, contains 0.784 grams of ammonium sulfide and 17.8 grams of water. The percentage by mass of ammonium sulfide in the solution is V 20 % 5 Retry Entire Group 9 more group attempts remaining PA FS T G Cengage Learning Cengage Technical Support B [Review Topics] [References] Use the References to access important values if needed for this question. 6 MacBook Air F6 " Y H & 7 N 44 U J 4 8 M Dil FB 1 * K ( 9 %. VS OWLV2 X DD FO O ) O L command A P ? V Chapter n 334) option x Previous + 1: (1 [ ? 1 OWLV2X + S 1 Next> Relaunch to update Save and Exit } deletearrow_forwardThe active ingredient of Benadryl© Chesty Forte Cough Liquid is Guaiphenesin anexpectorant drug used to assist the expectoration ('bringing up') of phlegm from theairways in acute respiratory tract infections. A 200.0 mL bottle of Benadryl© ChestyForte Cough contains 4.00 g of Guaiphenesin (C10H14O4). i. If the recommended dose for a child is 150 mg of Guaiphenesin. What volume of thecough liquid should be administered?arrow_forwardFe3+ in 0.725 M Fe2(SO4)3arrow_forward
- CI H₂O CN 1. ONa doon it OCH3 2. 3. H3O+ +H H CH3arrow_forwardd q eq _ch06.doc # Complete the table below for calculating the molecular weight of the compound 2,2,3,3-tetramethylbutane, shown in the model. E go FL $ Atom Atomic Weight C 12.01 amu/atom X H 1.01 amu/atom x molecular weight of 2,2,3,3-tetramethylbutane = Submit Answer R ball & stick labels v -+ F 288 %6 [Review Topics] [References] Use the References to access important values if needed for this question. 5 T G Retry Entire Group FO B 6 224 16 H # Atoms 7 N & atoms atoms = . 9 more group attempts remaining U = J * 8 Weight in compound amu amu M FB amu 1 K ( 9 DD F9 O ) 0 L F10 P A ¡ Previous Next> Email Instructor + 1 11 1 Save and Exit Show All 1 X deletearrow_forwardPlease answer a and barrow_forward
- What do these two tests indicate about the unknown molecule?arrow_forwardFill in the tables with the missing informationarrow_forwardCalculate the protein count of 100 g of a food, with a total amount of proteinof 20 g and the following composition of amino acids for each g of protein: 200 mg oflysine, 125 mg valine, 182 mg glycine, 290 mg proline, and 43 mg leucine. TwoWhole eggs (100 g) contain 12.8 g of protein and 86 mg of leucine, 70 mg of lysine,66 mg of valine for every g of protein.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY