
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:3. Predict the major products of the following reactions. Include stereochemistry where
appropriate.
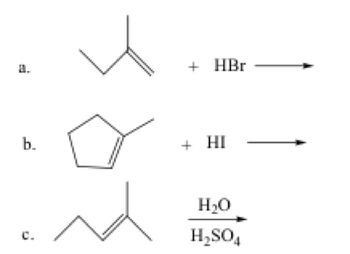
Transcribed Image Text:b.
+ HBr
+ HI
H₂O
H₂SO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1What is the molarity of an HCl solution if 20.0 mL is neutralized in a titration by 32.0 mL of 0.500 M NaOH 2. How many moles of HCl are there in 25.0 mL of 1.50 M HCl solution?arrow_forwardAa.23.arrow_forwardYou have a standard stock 1.65 M solution of NaH₂PO4. For an experiment you require 236 mL of a 0.82 M solution of NaH2PO4. How much of the 1.65 M solution do you need? Write your answer to 3 significant figures in mL.arrow_forward
- Part A Constants I Periodic A 0.5880 g sample of impure magnesium hydroxide is dissolved in 102.0 mL of 0.2044 M HCl solution. The excess acid then needs 19.88 mL of 0.1039 M NaOH for neutralization. Calculate the percent by mass of magnesium hydroxide in the sample, assuming that it is the only substance reacting with the HCl solution. Express your answer using four significant figures. ΜΕ ΑΣΦ Submit Request Answer Provide Feedback LG Backspace F12 1 ? % Delete End Down Next >arrow_forwardPlease help me answer questions 1 and 2. (Questions attached below) 1. When you arrive at the lab, 2.0 M H2SO4(aq) and 2.0 M HC2H3O2(aq) will be present. Youwill need to dilute these solutions using beakers and graduated cylinders from your drawerand DI water.i. Calculate the volume of the 2.0 M HC2H3O2(aq) and DI water needed to create 50.0 mLof 1.05 M HC2H3O2(aq).ii. Calculate the volume of the 2.0 M H2SO4(aq) and DI water needed to create 50.0 mL of0.75 M H2SO4(aq). 2. A 48.33-mL sample of 0.150 M NaBr is titrated into 50.00 mL of 0.145 M AgNO3. Bothsolutions had the same initial and final temperatures. The reaction occurred in a calorimeterwith a known heat capacity and produced 1546.03 J of heat.i. Write a balanced equation that is occurring.ii. Determine the theoretical yield of the solid.iii. Calculate the enthalpy of the reaction (kJ/mole of precipitate).arrow_forwardBy pipet, 9.00 mL of a 0.823 M stock solution of potassium permanganate (KMNO4) was transferred to a 50.00-mL volumetric flask and diluted to the calibration mark. Determine the molarity of the resulting solution. HA Value Unitsarrow_forward
- Calculate the volume of 0.164 M HNO3 required to neutralize 25.0 mL of 0.250 M NaOH to the correct number of significant figuresarrow_forwardDuring a titration the following data was collected. A 50.0 mL portion of H2SO4 was titrated with 0.500 M KOH. 200. mL of the base was needed to neutralize the acid. Calculate the molarity of the acidarrow_forwardIf 53.0 g of KClO3 was transferred into 125.0 g of water at 30.0 celcius. how many grams of KClO3 will percipitate? g solute at 30.0 celcius is 10 gramsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY