
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Question
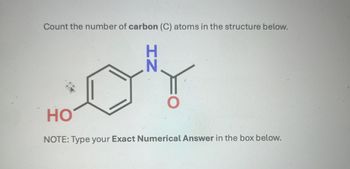
Transcribed Image Text:Count the number of carbon (C) atoms in the structure below.
NH
HO
NOTE: Type your Exact Numerical Answer in the box below.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Please answer question 20 Part Aarrow_forward3. Classify the following reactions as one of the following types: addition, substitution, hydrogenation, halogenation, or combustion. Write the names and the structures for all reactants and products. (6 Marks) a) Ethyne + Cl2 b) CH2=CH2 +arrow_forwardSelect the single best answer. Predict which compound has the higher melting point.arrow_forward
- For some questions you will need to use the special periodic table attached in the images! Treat Je, Qu, Ap, and Bg as NONMETALS! Classify each of the following alcohols as primary (1°), secondary (2°), or tertiary (3°)? Look at attached images (Please show work so I can understand going forward)arrow_forwardSee image belowarrow_forward4arrow_forward
- (Picture attached) Functional Groups: 1. Benzene 2. Halogen 3. Carboxyl 4. Hydroxyl Identify the functional groups that the 2 molecules contain. Note: each functional group can be used more than once. Put in numerical order with no space. Sucarlose = Ibuprofen =arrow_forwardfor molecule 2,6-dimethyloct-2-ene draw/print the structural formula of your molecule (expanded or condensed) and indicate the functional groups present by highlighting, circling, or color-coding each and labeling the group. what is the The molecular formula The molar mass (use atomic masses from the periodic table to the hundredths place for each element)arrow_forwardThis last question is different. Just because it is different doesn't make it hard. It's actually very easy. However, you DO have to know simple properties of molecules. Using simple tests, describe one single thing you can do to each pair of compounds in order to tell which is which. ALL YOU ARE ALLOWED TO USE IS: Matches, Water, Dilute NaHCO3, Dilute HCI, and Dilute NaOH. To help you, here is an example of the question, as well as the approach/thought process: "DESCRIBE ONE SINGLE TEST YOU COULD USE TO DIFFERENTIATE BETWEEN NaCl AND C12H2m011. ANS: the one test you can perform involves matches. NaCl is inorganic and will not burn, whereas C12H22O1 WILL burn because it is organic." Simple, yes? Now apply similar thinking to these pairs. OH NH2 & OH ОН b. & OH CODH &arrow_forward
- The condensed formula for the molecule 2,5-dimethylheptane is CH3CH (CH3)CH2CH2CH(CH3)CH2CH3. Part: 0/2 Part 1 of 2 Draw the structural formula for this molecule. Click and drag to start drawing a structure. ☐ X A D: 貝arrow_forward1. hexyne 2. hexane 3. cyclohexene 4. Organic Compounds 5. cyclohexane 6. benzene 7. 3,3-dimethylbut-1-ene 8. ◆ The four molecules listed above that are isomers of C6H12 are numbered (Record all four digits of your answer in lowest-to-highest numerical order) ◆arrow_forward4.61 What is actually measured by the octane ratings of different grades of gasoline?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning  Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning