
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
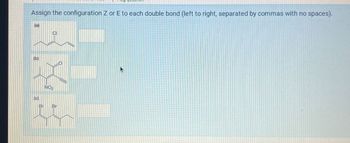
Transcribed Image Text:Assign the configuration Z or E to each double bond (left to right, separated by commas with no spaces).
(B)
(b)
(c)
NO
CI
Br
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- The unsaturation number or degree of unsaturation (U) can be used to determine the number of rings and multiple bonds in a compound from its molecular formula. Given a structure, you can determine the number of hydrogens without having to count them explicitly. Consider three compounds and their degree of unsaturation. (a) A compound A has the molecular formula C7H13ClN2OC7H13ClN2O. How many rings and/or π bonds does it contain?arrow_forwardRegarding the following condensed structures, what are their bond-line structures?arrow_forwardDraw a bond line structure for (ch3)2cchchoarrow_forward
- Indicate the direction of the expected polarity of each of the bonds shown below: (a) CH3- NH2 (b) CH3- Liarrow_forwardLinoleic acid (below) is an essential fatty acid found in many vegetable oils, such as soy, peanut, and cottonseed. A key structural feature of the molecule is the cis orientation around its two double bonds, where R1 and R2 represent two different groups that form the rest of the molecule. R, CH2 `H H' (a) How many different compounds are possible, changing only the cis/trans arrangements around these two double bonds? (b) How many are possible for a similar compound with three double bonds? R3. .CH2 R4 `H H `H H'arrow_forwardCircle the most basic atom or group of atoms in each of the following molecules:arrow_forward
- Which of the following bond-line structures corresponds to the Newman projection shown? Br J... (A) Br I H. Br "Ω (B) CH3 CH3 CI H Br (C) Br CI (D)arrow_forwardDraw all of the resonance structures for each of the following species. Be sure to include the curved arrows that indicate which pairs of electrons are shifted in going from one resonance structure to the next. Draw the resonance hybrid of each species. (a) ОН (b) (c) H3Carrow_forwardThe answer for this problem is a & b. But I dont understand or know why c isn't one of the correct answers. Which of the following has at least one C-O single bond?(more than one answer may be possible) a) H2CO3 b) HCO3- c) CO32-arrow_forward
- please answer all questions.arrow_forwardQ2. Draw a zig-zag representation for each molecule below showing all valence electrons on atoms other than carbon and hydrogen and include any formal charges should there be any (none of the compounds contain a ring of atoms).(a) CH3CH=CHCH(OH)CH3(b) (CH3)2CHCH2CO2CH2CH3arrow_forwardWhat is the correct IUPAC name for HBRO(aq)? (1) (II) (II) (IV) tri- tetra- octa- penta- hepta- mono- di- hexa- hypobromic oxide охудen bromous hydrogen bromine hydrobromous hypobromous bromic hydrate acid Deletearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning