
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
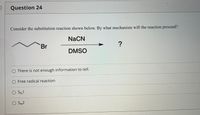
Transcribed Image Text:Question 24
Consider the substitution reaction shown below. By what mechanism will the reaction proceed?
NaCN
Br
DMSO
O There is not enough information to tell.
O Free radical reaction
O SN1
O SN2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CHEM 120 Recitation RC7 Part IV: Provide the mechanism and product for the reaction shown below. Provide the energy diagram for the reaction shown above. Label enthalpy of the reaction and activation energy. CHEM 120 Recitation Part IV: 1. Provide the mechanism and product for the reaction shown below. Br DMF ONa RC7 2. Provide the energy diagram for the reaction shown above. Label enthalpy of the reaction and activation energy.arrow_forwardWhich species does the first transition state of the overall reaction most ressemble? What kind of transition state is this (late or early)?arrow_forwardUsing the correct catalysts and solvents, what are the likely product outcomes of step 1 and 3 of the following reaction?arrow_forward
- 5. w mexico What is the likely mechanism of this reaction? ew mexico Br A. E1cb B. E1 C. E2 D. None of the choices are correct. NaNH, DMF 6. I can answer question #5 because I know that: A. The solvent is polar protic, so a cation is easily formed B. The base is ionic, and so is a strong base C. The product follows Zaitsev's Rule D. The stability of the conjugate base allows for easy deprotonation as a first steparrow_forwardCan you help me figure this out please?arrow_forwardWhich of the following is the most stable free radical? (Note: if a species has resonance stabilization, only one resonance structure is drawn for it.) A C O A ов C OD B Darrow_forward
- Choose one.arrow_forwardWhat is the kinetically controlled product in the following reaction? A Br OA. A OB. B О с. с OD. D B Br HBr ?? C Br Br Darrow_forwardQ1A: step named A, B, C, D as one of either (Termination, Propagation, Initiation) Q1B: For radical reaction, match the intermediate 1,2,3,4 to the correct possible intermediate W,X,Y or Zarrow_forward
- In an elimination reaction, the rate did not change when the base concentration was increased 3 times. What kind of mechanism did it follow? SN1 SN2 E1 E2arrow_forwardWhich of the following represents the transition state of the rate- determining step in the substitution reaction between tert-butyl bromide and methanol? H3C CH, H,C CH, Br sOc--------Br 1 H3C 2 H3C CH: CH3 H3C CH, Br -Br а. 1 b. 2 с. 3 d. 4 a O barrow_forwardWhat will be plausible mechanism of the given reaction below?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY