
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
1d.
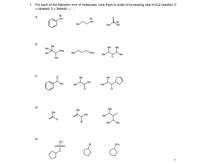
Transcribed Image Text:1. For each of the followina sets of molecules, rank them in order of increasing rate of SN2 reaction (1
= slowest; 3 = fastest). (:' : -
а.
NH
NH
Me
Me
NH
b.
Me
Me
Me
Me.
SNa
Me
Me
ONa
Me
Me
Li
Me
С.
Me
Me
Me
"Ме
Me
Me
CI
CI
d.
Me
Me
Me
Me
Me
Br
Me
Me
е.
Br
NH2
O=S=C
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- a gallon of water weighs about 4.9 kg. how many gallons of water are there in 29.4kg?arrow_forwardGold is a very dense metal with a density of 18.3 g/cm3, what is the volume of a 0.255 kg gold sample?arrow_forwardYour 50 mL beaker has a mass of 6.29 g. You add 10.00 mL to the 50 mL beaker and now the total mass is 16.79 g. What is the density of the solution in g/mL?arrow_forward
- 2896 ng to mgarrow_forward5. An irregularly shaped piece of metal is placed in a graduated cylinder containing 15.0mL of water. After the metal was added, the water level rose to 27.6mL. The piece of metal was weighed and the mass was found to be 0.53323kg. Determine the density of the piece of metal in g/cm'. 3arrow_forwardA particular paint has a density of O.914g/cm3. You need to cover a wall that is 7.6m long and 2.74m high with a paint layer 0.13mm thick. What volume of paint (in litres) is required? What is the mass (in grams ) of the paint layer?arrow_forward
- A chemist makes 730.mL of potassium dichromate K2Cr2O7 working solution by adding distilled water to 220.mL of a 0.139molL stock solution of potassium dichromate in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits.arrow_forwardA doctor orders 2.0 mgmg of morphine. The vial of morphine on hand is 30. mgmg per 6.0 mLmL . How many milliliters of morphine should you administer to the patient?arrow_forwardBulk platinum cost about $1158 dollars per ounce (28.35 g = 1 ounce) how much is this per gram?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY