
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
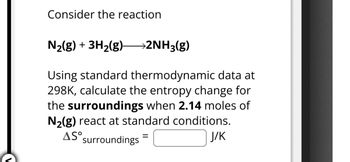
Transcribed Image Text:Consider the reaction
N2(g) + 3H2(g) >2NH3(g)
Using standard thermodynamic data at
298K, calculate the entropy change for
the surroundings when 2.14 moles of
N2(g) react at standard conditions.
AS° surroundings
=
J/K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- What is the entropy change of a gas in J/K (to 2 decimal points) when one mole of a perfect gas reversibly expands from 0.36 m3 to 2.42 m3?arrow_forwardConsider the reaction: 3FE203(s) + H2(g)- →2F@304(s) + H20(g) Using standard thermodynamic data at 298K, calculate the free energy change when 2.39 moles of Fe203(s) react at standard conditions. AG°rxn = kJarrow_forwardConsider the reaction 4-entro 4HС(g) + O2(g)- →2H2O(g) + 2Cl2(g) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 1.50 moles of HCl(g) react at standard conditions. AS surroundings J/Karrow_forward
- The decomposition of nitrogen pentoxide in the reaction 2N₂O5 (s)→ 4NO2(g) + O2(g) is endothermic with AH = +110 kJ/mol. Even so, this reaction is spontaneous for temperatures above 230 K because the change of a solid into a gas is a large increase of entropy.arrow_forwardCalculate ASsurr and ASuniverse for a reaction. Consider the reaction H₂S(g) + 2H₂O(1)→→3H₂(g) + SO₂(g) Using standard thermodynamic data (in the Chemistry References), calculate the entropy change of the surroundings and the universe at 25°C. AS surroundings = ASuniverse J/K J/K where ASO = 294.7 J/K rxnarrow_forwardConsider the reaction: 4HCl(g) + O₂(g) → 2H₂O(g) + 2Cl₂ (g) Using standard absolute entropies at 298 K, calculate the entropy change for the system when 1.69 moles of HCl(g) react at standard conditions. AS⁰ Substance So (J/K mol) HCl(g) 186.9 O2(g) 205.1 H₂O(g) 188.8 Cl₂ (g) 223.1 system = J/Karrow_forward
- Consider the reaction: Fe2O3(s) + 2Al(s) → Al2O3 (s) + 2Fe(s) Using standard thermodynamic data at 298 K, calculate the entropy change for the surroundings when 2.00 moles of Fe₂O3(s) react at standard conditions. AS⁰ Substance AH (kJ/mol) Fe₂O3(s) -824.2 Al(s) 0.0 Al2O3(s) -1675.7 Fe(s) 0.0 surroundings J/Karrow_forwardConsider the reaction: 2BrF3(g) → Br2(g) + 3F2 (g) Using standard thermodynamic data at 298 K, calculate the free energy change when 2.40 moles of BrF3 (g) react at standard conditions. Substance AG (kJ/mol) BrF3 (9) Br₂ (9) F₂ (g) AGTER Submit Answer kj -229.4 3.1 0.0 Retry Entire Group 9 more group attempts remainingarrow_forwardConsider the reaction HCI(g) + NH3(g)- →NH4Cl(s) Using standard thermodynamic data at 298K, calculate the entropy change for the surroundings when 2.34 moles of HCI(g) react at standard conditions. ASO surroundings = J/Karrow_forward
- [Review Topics] [References] Use the References to access important values if needed for this question. Consider the reaction: 2HBr(g) → H₂(g) + Br₂ (1) Using standard thermodynamic data at 298 K, calculate the free energy change when 2.01 moles of HBr(g) react at standard conditions. AGO rxn Substance AG (kJ/mol) HBr(g) H₂(g) Br₂ (1) = -53.5 0.0 0.0 KJ Retry Entire Group Submit Answer 9 more group attempts remainingarrow_forwardOne mole of an ideal monatomic gas is compressed from 2.0atm to 6.0 atm while being cooled from 400 K to 300 K. Calculate the values of ΔU, ΔH, and ΔS for the overall processarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY