
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
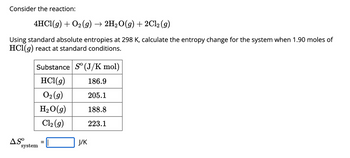
Transcribed Image Text:Consider the reaction:
4HCl(g) + O₂(g) → 2H₂O(g) + 2Cl₂ (g)
Using standard absolute entropies at 298 K, calculate the entropy change for the system when 1.90 moles of
HCl(g) react at standard conditions.
Substance So (J/K mol)
HCl(g)
186.9
O₂(g)
205.1
H₂O(g)
188.8
Cl₂(g)
223.1
ASO
system
=
J/K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the data in the table, calculate the standard entropy changes for the following reactions at 25°C. S(s) + O2(g) → S02(g) Species [J/(mol K)] S(s) 167.83 J/(mol K) O2(g) 205.15 MGCO3(s) → MgO(s) + CO2(g) SO,(g) 248.22 MGCO3(s) 65.7 J/(mol K) MgO(s) 27.0 CO28) 213.78 2 C,H,(g) + 7 O2g)→ 4 CO2(g) + 6 H2O(1) C;Hg) 229.16 J/(mol K) H,0() 69.95arrow_forwardAcetone, CH; COCH3 , boils at 56°C. The heat of vaporization of acetone at this temperature is 29.1 kJ/mol. What is the entropy change when 9 mol of liquid acetone vaporizes at 56°C?arrow_forwardStandard Entropy of Reaction Molecular systems tend to move spontaneously to a state of maximum randomness or disorder. Molecular randomness, or disorder, is called entropy and is denoted by the symbol S. As a state function, entropy change, A.S. depends only on initial and final states. AS has a positive value when disorder increases and a negative value when disorder decreases. The following conditions usually result in an increase in entropy: • a change of phase: solid-liquid-gas, an increase in the number of gas molecules, or • a solid dissolving to form a solution. Although the sign of the entropy change can be predicted as described above, the actual value of AS° must be calculated from the absolute entropy values. Sº, of the reactants and products: ASS° (products) - S° (reactants) ▼ Part A Predict the sign of the entropy change. AS°, for each of the reaction displayed. Drag the appropriate items to their respective bins. ▸ View Available Hint(s) Positive Submit H₂O(1) H₂O(g)…arrow_forward
- The decomposition of nitrogen pentoxide in the reaction 2N₂O5 (s)→ 4NO2(g) + O2(g) is endothermic with AH = +110 kJ/mol. Even so, this reaction is spontaneous for temperatures above 230 K because the change of a solid into a gas is a large increase of entropy.arrow_forwardCalculate ASsurr and ASuniverse for a reaction. Consider the reaction H₂S(g) + 2H₂O(1)→→3H₂(g) + SO₂(g) Using standard thermodynamic data (in the Chemistry References), calculate the entropy change of the surroundings and the universe at 25°C. AS surroundings = ASuniverse J/K J/K where ASO = 294.7 J/K rxnarrow_forwardConsider the reaction: 4HCl(g) + O₂(g) → 2H₂O(g) + 2Cl₂ (g) Using standard absolute entropies at 298 K, calculate the entropy change for the system when 1.69 moles of HCl(g) react at standard conditions. AS⁰ Substance So (J/K mol) HCl(g) 186.9 O2(g) 205.1 H₂O(g) 188.8 Cl₂ (g) 223.1 system = J/Karrow_forward
- For the following reaction (the detonation of nitroglycerin), is it spontaneous under standard conditions and how can you tell? 4 C4H5N3O9(l) (arrow >) 12 CO2(g) + 10 H2O(g) + 6 N2(g) + O2(g) ΔH = -5678 kJarrow_forwardThe decomposition of copper (1) oxide to its elements does not occur spontaneously at 375°C (AG = 140.0 kJ): Cu₂0 (s) 2 Cu (s) + 1/2O₂(g). It can be made to occur spontaneously by being coupled to the oxidation of solid carbon to form carbon monoxide gas at this same temperature (AG = -143.8 kJ): C(s) + 1/2O₂(g) → CO (g) What product(s) is/are produced as a result of coupling the two reactions? CO (g) 2 Cu (s) and 1/2 O₂ (g) 2 Cu (s) and CO (g) 2 Cu (s), 1/2 O2 (g), and CO (g) 1/2 0₂ (g)arrow_forwardGiven the following reaction, calculate the standard entropy at 25oC. 2NO(g) + O2(g) 2NO2(g) (Sof NO2 = 240.1 J mol-1K-1; Sof NO = 210.8 J mol-1K-1; Sof O2 = 205.1 J mol-1K-1)arrow_forward
- Consider the following reaction: C(graphite) + 1/2 O2 (g) → CO(g) Is it spontaneous under standard conditions at 298 K? Answer this question by calculating AS" (system), AS° (surroundings), and AS (universe). (Define reactants and products as the system.) S* (J/mol · K) A¡H° (kJ/mol) at 298 K (kJ/mol) Compound at 298 K C(s, graphite) 5.6 CO(g) 197.674 -110.525 CS2 (g) 237.8 116.7 CCL (e) 214.39 -128.4 Cl2 (g) 223.08 Si(s) 18.82 SİCL, (g) 330.86 -662.75 S(s, rhombic) 32.1 Br2 (e) 152.2 HBr(g) 198.70 -36.29 H2 (g) 130.7 H2O2 (e) 109.6 -187.78 O2 (g) 205.07 O yes O noarrow_forwardgiven rgetable of standard molar entropy values of each substance in the reaction below, what is the standard change in entropy, delta s, for the following reaction? 2CH3OH(g)+3OH2- 2CO2(g)+4H2O(g) Substance= CH3OH(g) S(J/mol•K) 240 Substance=O2(g) S(J/mol•K) 205 Substance=CO2(g) S(J/mol•K) 214 Substance=H2O(g) S(J/mol•k) 189 a. -352 J/K b. -1302 J/K c. 315 J/K d. 89 J/K E. 1830 J/Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY