
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Don't use AI.
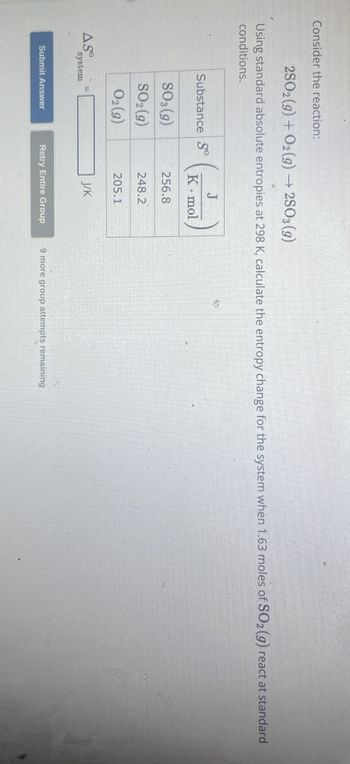
Transcribed Image Text:Consider the reaction:
2SO2(g) + O2(g) → 2SO3 (9)
Using standard absolute entropies at 298 K, calculate the entropy change for the system when 1.63 moles of SO2 (g) react at standard
conditions.
J
Substance So
K mol
SO3 (9)
256.8
SO2(g)
248.2
02 (9)
205.1
J/K
ASO
system
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- The synthesis of glucose directly from CO2 and H2O and the synthesis of proteins directly from amino acids are both non-spontaneous processes under standard conditions. Yet it is necessary for these to occur for life to exist. In light of the second law of thermodynamics, how can life exist?arrow_forwardLiquid water at 25C is introduced into an evacuated, insulated vessel. Identify the signs of the following thermodynamic functions for the process that occurs: H, S, Twater, Ssurr,, Suniv.arrow_forwardLimestone is predominantly CaCO3, which can undergo the reaction CaCO3(s)CaO(s)+CO2(g). We know from experience that this reaction is not spontaneous, yet S for the reaction is positive. How can the second law of thermodynamics explain that this reaction is not spontaneous?arrow_forward
- The standard molar entropy of methanol vapor, CH3OH(g), is 239.8 J K1 mol-1. (a) Calculate the entropy change for the vaporization of 1 mol methanol (use data from Table 16.1 or Appendix J). (b) Calculate the enthalpy of vaporization of methanol, assuming that rS doesnt depend on temperature and taking the boiling point of methanol to be 64.6C.arrow_forwardExplain why absolute entropies can be measured.arrow_forwardA key component in many chemical engineering designs is the separation of mixtures of chemicals. (a) What happens to the entropy of the system when a chemical mixture is separated? (b) Are designs for chemical separation more likely to rely on spontaneous or nonspontaneous processes?arrow_forward
- It has been demonstrated that buckminsterfullerene (C60), another allotrope of carbon (Section 2.3), may be converted into diamond at room temperature and 20,000 atmospheres pressure (about 2 GPa). The standard enthalpy of formation, fH, for buckminsterfullerene is 2320 kJ/mol at 298.2 K. a. Calculate rH for the conversion of C60 to diamond at standard state conditions and 2982 K. b. Assuming that the standard entropy per mole of carbon in both C60 and diamond is comparable (both about 23 J/K mol), is the conversion of C60 to diamond product-favoredat room temperature?arrow_forwardAccording to Lambert, leaves lying in the yard and playing cards that are in disarray on a table have not undergone an increase in their thermodynamic entropy. Suggest another reason why leaves and playing cards may not be a good analogy for the entropy of a system containing, for example, only H2O molecules or only O2 molecules.arrow_forwardChemists and engineers who design nuclear power plants have to worry about high-temperature reactions because it is possible for water to decompose. (a) Under what conditions does this reaction occur spontaneously? 2H2O(g) 2H2(g) + O2(g) (b) Under conditions where the decomposition of water is spontaneous, do nuclear engineers have to worry about an oxygen/hydrogen explosion? Justify your answer.arrow_forward
- What is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the synthesis of ammonia? 3H2(g) + N2(g) 2NH3(g)arrow_forwardThe evaporation of one mole of water at 298 K has a standard free allergy change of 8.58 kJ. H2O(l)H2O(g)G298=8.58kJ (a) Is the evaporation of water under standard thermodynamic conditions spontaneous?. (b) Determine the equilibrium constant, KP, for this physical process. (c) By calculating G, determine if the evaporation of water at 298 K is spontaneous when the partial pressure of water, PH2O, is 0.011 atm.. (d) If the evaporation of water were always nonspontaneous at room temperature, wet laundry would never dry when placed outside. In order for laundry to dry, what must be the value of PH2O in the air?arrow_forwardWhen ammonium chloride is added to water and stirred, it dissolves spontaneously and the resulting solution feels cold. Without doing any calculations, deduce the signs of G, H, and S for this process, and justify your choices.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning