
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
please help me answer both questions
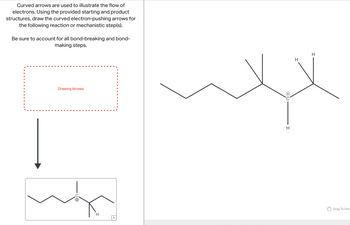
Transcribed Image Text:Curved arrows are used to illustrate the flow of
electrons. Using the provided starting and product
structures, draw the curved electron-pushing arrows for
the following reaction or mechanistic step(s).
Be sure to account for all bond-breaking and bond-
making steps.
Drawing Arrows
-CO
H
Q
H
H
+0
H
Drag To Pan
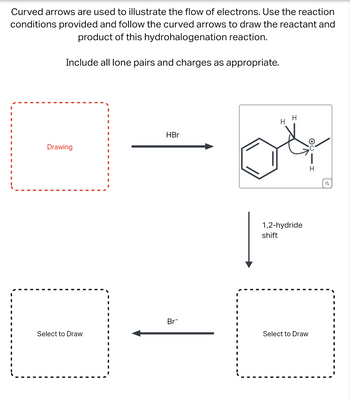
Transcribed Image Text:Curved arrows are used to illustrate the flow of electrons. Use the reaction
conditions provided and follow the curved arrows to draw the reactant and
product of this hydrohalogenation reaction.
Include all lone pairs and charges as appropriate.
H
H
Drawing
HBr
1,2-hydride
shift
Br¯
Select to Draw
Select to Draw
H
Q
I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the first step of this reaction sequence. Be sure to account for all bond-breaking and bond- making steps. I H BH₂ H B H Select to Add Arrows > H Bll H BH₂arrow_forwardFor each of the reaction below, draw the curved arrows and lone pairs of electrons to show the mechanism. Predict which side of the reaction will be favoured under equilibrium conditions. Give a brief explanation of your prediction. NーHarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or to show the interconversion between resonance hybrid contributors. Be sure to account for all bond-breaking and bond-making steps.arrow_forward
- Curved arrows are used to illustrate the flow of electrons, Using the provided starting and product structures, draw the curved electon-pushing arrows for the following reaction or machnistic step(s). Be sure to account for all bond-breaking and bond-making stepsarrow_forwardDraw the mechanism and the energy diagram for the reaction shown below. Include any resonance structures for the intermediates of the reaction. H3O+arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H Drawing Arrows NaOH, H₂O heat Na → H ધ H H H HE H. O H H :I:O :9: H Na + Harrow_forward
- Add curved arrows to show the forming and breaking of bonds in the reaction below. C с Ċ Add/Remove steparrow_forwardUse curved arrows to keep track of the electrons shown in the following reaction. 1-iodomethylcyclohexane →→ tertiary cation + iodide anionarrow_forwardDraw both resonance structures of the most stable carbocation intermediate in the reaction shown HCI - You do not have to consider stereochemistry. -Do not include anionic counter-1ons, e.g., I, in your answer. - Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. - Separate resonance structures using the symbol from the drop-down menu. y自ノ ee P. opy aste [F CHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Na O Na O co Select to Add Arrows H H H po ² Select to Add Arrows 0:0arrow_forwardDraw both resonance structures of the most stable carbocation ntermediate in the reaction shown. +HBr • You do not have to consider stereochemistry. Do not include anionic counter-1ons, e.g., I, in your answer. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate resonance structures using the → symbol from the drop-down menu, P. opy aste C. 000▼[片 vate Windowsarrow_forwardGiven the reactant Br−Br, add curved arrows to show homolytic bond cleavage, then draw the expected product. Be sure to add any charges and nonbonding electrons that result from the cleavage.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning