
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Consider the pentapeptide Ile-Lys-Asp-Phe-Gly and answer the following (use the pKa values in the table):
1. Draw the structure of the predominant form of the pentapeptide at pH = 12.
2. Draw the structure of the Zwitterion form of the pentapeptide.
3. What is the IpH of the peptide?
Handwritten solution
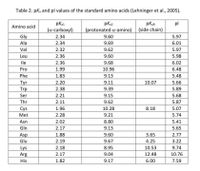
Transcribed Image Text:Table 2. pka and pl values of the standard amino acids (Lehninger et al., 2005).
pKaR
(side chain)
pKa1
pKa2
pl
Amino acid
(а-carboxyl)
(protonated a-amino)
Gly
2.34
9.60
5.97
Ala
2.34
9.69
6.01
Val
2.32
9.62
5.97
Leu
2.36
9.60
5.98
lle
2.36
9.68
6.02
Pro
1.99
10.96
6.48
Phe
1.83
9.13
5.48
5.66
Тyr
Trp
2.20
9.11
10.07
2.38
9.39
5.89
Ser
2.21
9.15
5.68
Thr
2.11
9.62
5.87
Сys
1.96
10.28
8.18
5.07
Met
2.28
9.21
5.74
Asn
2.02
8.80
5.41
Gln
2.17
9.13
5.65
Asp
1.88
9.60
3.65
2.77
Glu
2.19
9.67
4.25
3.22
2.18
Lys
Arg
8.95
10.53
9.74
2.17
9.04
12.48
10.76
His
1.82
9.17
6.00
7.59
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 9 steps with 7 images

Knowledge Booster
Similar questions
- The R group of the amino acid Asp contains a carboxyl group with a pKa of 3.7. But the pka of the alpha-carboxyl group in Asp is 1.9. Which of the following. explanations could reasonably account for this difference? 3.7 is the isoelectric pH, which is estimated by averaging the pka of the alpha- carboxyl group with the much higher pKa of the alpha-amino group The R group of Asp also contains an NH2 group, which is less acidic than a carboxylic acid The conjugate base of the alpha-carboxyl could be stabilized by an attractive ionic interaction with the nearby alpha-amino group, which is protonated at low pH Water has better access to the R group than to the alpha-carboxyl group, so it more effectively solvates the charged form of the R group and stabilizes itarrow_forwardPeptides can be separated using an ion-exchange column based on their isoelectric (pl) values. At which pH values would two different peptides, one with a pl of 5.6 and the other with a pl of 9.1, bind to a cation- and anion-exchange column? Each peptide may be capable of binding to each column at more than one pH value. anion-exchange column at pH = 3.1 pH = 7.2 pH = 10.6 ****** cation-exchange column at pH = 3.1 pH = 7.2 PH =10.6 Answer Bank peptide B pl = 9.1 peptide A pl = 5.6arrow_forwardDraw a tetra peptide of Ser-His-Leu-Tyr at pH=1arrow_forward
- The RGD peptide is critical for the establishment of the extracellular matrix of animal cells. a. Draw the structure of the RGD peptide at physiological pH (pH = 7). b. What is the charge of this peptide at physiological pH? At high pH (pH> 13)? Low pH (pH < 3)?arrow_forwardReport the isoelectric point of the peptide, Gly-Asp-Lys-Ile?arrow_forwardAn unknown amino acid labeled KEMISTRI has an IpH value equal to 5.41. If its pKa1 is equal to 2.03, calculate the pKa2 of KEMISTRI. Show complete solutions. Based on the table below, what is the probable identity of KEMISTRI?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY