
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
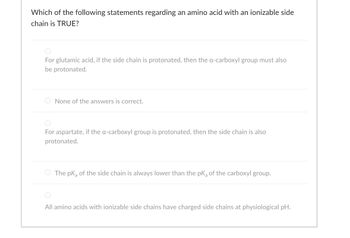
Transcribed Image Text:Which of the following statements regarding an amino acid with an ionizable side
chain is TRUE?
For glutamic acid, if the side chain is protonated, then the a-carboxyl group must also
be protonated.
None of the answers is correct.
For aspartate, if the a-carboxyl group is protonated, then the side chain is also
protonated.
The pk of the side chain is always lower than the pk of the carboxyl group.
All amino acids with ionizable side chains have charged side chains at physiological pH.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the predominant form of glutamic acid at pH = 8.4. The pka of the side chain is 4.1. Include proper stereochemistry. HO H₂N, pH = 8.4 Drawing OH F Drarrow_forwardDoarrow_forward▼22. From each description, state whether the unknown solution contains an amino acid, a peptide, or a protein. a. An unknown solution gives a negative biuret test, but a positive xanthoproteic test. What may the unknown solution contain?arrow_forward
- considering the following peptides: Phe-Tyr-Ala-Lys-Glu-Asp the amino acid constituents that will have a protonated amino group at pH 7 is: a- lys and asp only b-phe, lys, and asp only c- phe and lys only d- lys onlyarrow_forwardWhat is the pI for the polypeptide ALRHEN? Use pKa of 8 for the N-terminus and 4 for the C-terminus.arrow_forwardAnswer the questions regarding the tripeptide shown. Macmillan Learning H₂N-CH-C-N-CH-C-N-CH-C H₂C CH Ovaline CH3 threonine Ocysteine serine H Which amino acid is the C-terminal amino acid? CHOH H CH₂ CH3 SH What is the name of this tripeptide, using the three-letter amino acid abbreviations? Capitalize the each abbreviation. m 10 of 22 > Draw the individual amino acids that form this tripeptide. Draw the zwitterion forms. More Rings Draw Select NHO ||| || / GOarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY