
Chemistry: Matter and Change
1st Edition
ISBN: 9780078746376
Author: Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher: Glencoe/McGraw-Hill School Pub Co
expand_more
expand_more
format_list_bulleted
Question
None
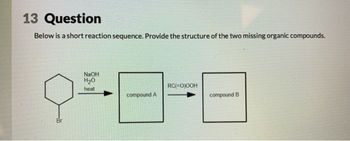
Transcribed Image Text:13 Question
Below is a short reaction sequence. Provide the structure of the two missing organic compounds.
Br
NaOH
H₂O
heat
RC(=O)OOH
compound A
compound B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Ff.179.arrow_forwardBelow is a short reaction sequence. Provide the structure of the two missing organic compounds.arrow_forwardBelow is a short reaction sequence. Provide the structure of the two missing organic compounds. 1. Hg(OAC2 CH3OH 2. NABH4 NaOH H20 heat compound A compound B Brarrow_forward
- Draw a structural formula for the major organic product(s) of the reaction shown below. `NH2 1. CH3! (еxcess) 2. Ag20, H2O, heatarrow_forwardComplete the reaction of propylene oxide with methylaminearrow_forwardWrite the product or products that will be formed as a result of the reactions given in each line below.arrow_forward
- Write down the common (not IUPAC) names of the organic molecules that would be released if this molecule were hydrolyzed: CH2−O−C—(CH2);–CH=CH–CH2–CH=CH—(CH2)4—CH3 CH-O-C-(CH2)14-CH3 O 11 CH2−O−C— (CH2)14 — CH3 Separate each name with a comma. You will find useful information in the ALEKS Data resource. 1 010 Continue O a X 000 Y F8 F9 Submiarrow_forwardCH3 CH3 H cyclopentamine The synthesis of cyclopentamine, an amphetamine-like central nervous system stimulant, from materials of five carbons or fewer involves several steps, one of which is shown below. Draw the structure(s) of the major organic product(s) of the following reaction: Mg, dry ether Br productarrow_forwardPredict the structure of the reactant or product in the following reactionsarrow_forward
- An important step in one synthesis of carboxylic acids is the deprotonation of diethyl malonate and its alkyl-substituted derivative: Base CH;CH2O OCH,CH3 CH;CH,0 OCH2CH3 H2 Diethyl malonate Base CH;CH,0 °C `OCH,CH3 CH;CH,O OCH,CH3 R Alkyl substituted diethyl malonate NaOH can deprotonate diethyl malonate effectively, but NaOC(CH3)3 is typically used to deprotonate the alkyl-substituted derivative. Explain why.arrow_forwardIn each of the following reactions, two possible organic products can be formed. Draw both organic products in each case and then circle the one formed in greatest quantity in each case. HC (a) 1) NaH, 2) acid (b) CH,CH,OH (c) CH,CH,OH NH2 (d) Oarrow_forwardProvide a step-by-step mechanism for the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co