
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:10:01 1
Question 3 of 25
Submit
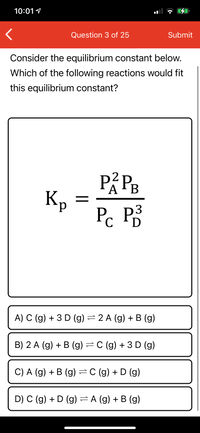
Consider the equilibrium constant below.
Which of the following reactions would fit
this equilibrium constant?
Кр
P. P3
D
A) C (g) + 3 D (g) =2 A (g) + B (g)
B) 2 A (g) + B (g) =C (g) + 3D (g)
C) A (g) + B (g) =C (g) + D (g)
D) C (g) + D (g) = A (g) + B (g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Express the equilibrium constant (K) for the chemical equations. (Note the physical states) A. CH3OH (g) ↔ CO (g) + 2 H2 (g) B. C3H8 (g) + 5 O2 (g) ↔ 3 CO 2 (g) + 4 H2O (g) C. SbCl5 (g) ↔ SbCl3 (g) + Cl2 (g)arrow_forwardWhat is the correct equilibrium constant expression for the balanced reaction shown below? PC13 (9) + PBr3 (9) PCl2Br (g) + PCIBr2(g) [PCl3] [PBr3] [PCl₂ Br][PCIBr₂] [PCIBr₂][PCl₂Br] [PCl3] [PBr3] [PCIBr₂][PCl3][PBr3] [PCl₂Br] [PCIBr₂]²[PCl₂Br]² [PCl3] [PBr3] [PCl3][PBr3] [PCIBr₂]²[PCl₂ Br]² O a. a O b.b OC.C O d.d O e.e a b C d earrow_forwardConsider the following reaction: 2CO (g) + O2 (g) ⇒ 2CO2 (g). In a 2.00 L vessel, 0.5 moles of carbon monoxide gas reacts with 0.5 moles of oxygen gas. Given that K = 2.2 × 10-22, what are the concentrations of all species at equilibrium? The concentration of CO at equilibrium is The concentration of O2 at equilibrium is The concentration of CO2 at equilibrium isarrow_forward
- Enter your answer in the provided box. Consider the following reaction at a high temperature. Br2(g) 2Br(g) When 1.30 moles of Br2 are put in a 0.710-L flask, 1.90 percent of the Br2 undergoes dissociation. Calculate the equilibrium constant Ke for the reaction.arrow_forwardFor the balanced reaction equation given below, 0.500 moles of H2 and 0.500 moles of I2 are placed in a 10.0 L reaction vessel. At equilibrium the concentration of HI was measure to be 0.078 M. Determine Kc for this reaction. H2(g) + I2(g) ↔ 2HI(g)arrow_forward@quinnipiac.edu doesn't allow editing on a Mac. To learn more, contact your admin about your Office plan. 4. What is the correct equilibrium constant (K.) expression for the following reaction? Fe2O3(s) + 3H2(g) 2 2Fe(s) + 3H20(g)arrow_forward
- For the following reaction and its equilibrium constant, determine whether the position of equilibrium lies towards reactants, towards products, or somewhere reasonably close to the middle (intermediate). NH3(aq) + HBrO(aq) ⇒ NH4*(aq) + BrO (aq) K = 5.0arrow_forwardAt a certain temperature, the following reactions have the equilibrium constants shown. S(s) + O2(g) ⇆ SO2(g). Kc1 = 4.2 × 1052 2S(s) + 3O2(g) ⇆ 2SO3(g). Kc2 = 9.8 × 10128 Calculate the equilibrium constant, Kc, for the following reaction at that temperature. (in scientic notation) 2SO2(g) + O2(g) ⇆ 2SO3(g)arrow_forwardConsider the general reaction below. 2A(g) + B₂(g) = 2AB(g) K = 6.4 x 10-6 @ 298 K The reaction was initiated by combining 0.70 mol A and 0.90 mol B2 in a 2.0 L container. Calculate the equilibrium concentrations of the components. What is the equilibrium concentration of AB? 10[? ] [AB] = [?] x 10¹ Marrow_forward
- What is the equilibrium-constant expression for the following reaction?N2O4 (g) <--> 2NO2 (g)arrow_forwardConsider the reaction 2 A + B <=> 3C + D. 3.00 moles A and 2.00 moles B was placed in a 2.00 L flask and allowed to reach equilibrium. At equilibrium the concentration of D was 0.500 M. Calculate Kc for this reaction.arrow_forward6. Give the equilibrium constant for the following reaction. 2PB1, (g) + 3Cl,(s) 2PCI;(g) + 3Br,(g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY