
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
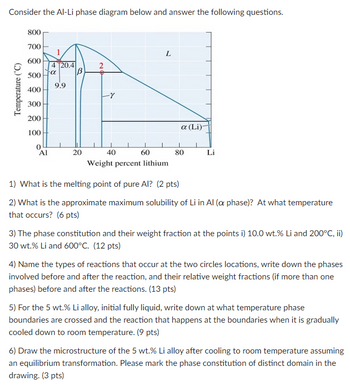
Transcribed Image Text:Consider the Al-Li phase diagram below and answer the following questions.
800
Temperature (°C)
700
L
600
4 20.4
2
α
500
β
9.9
400
-Y
300
200
100
0
ΑΙ
20
40
60
α (Li)-
80
Li
Weight percent lithium
1) What is the melting point of pure Al? (2 pts)
2) What is the approximate maximum solubility of Li in Al (a phase)? At what temperature
that occurs? (6 pts)
3) The phase constitution and their weight fraction at the points i) 10.0 wt.% Li and 200°C, ii)
30 wt.% Li and 600°C. (12 pts)
4) Name the types of reactions that occur at the two circles locations, write down the phases
involved before and after the reaction, and their relative weight fractions (if more than one
phases) before and after the reactions. (13 pts)
5) For the 5 wt.% Li alloy, initial fully liquid, write down at what temperature phase
boundaries are crossed and the reaction that happens at the boundaries when it is gradually
cooled down to room temperature. (9 pts)
6) Draw the microstructure of the 5 wt.% Li alloy after cooling to room temperature assuming
an equilibrium transformation. Please mark the phase constitution of distinct domain in the
drawing. (3 pts)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 1 Al-Li Aerospace Alloys When a small amount of Li is added into Al to create an Al-Li alloy, the two elements typically do not mix homogeneously. Instead, most of the material is nearly-pure Al, while some small regions are Li-rich.¹ (These small regions are called precipitates and they are responsible for giving Al-Li alloys much better properties for aerospace applications relative to pure Al. We'll learn more about the alloying process and about precipitates later in the course.) The Li-rich regions have the chemical formula Al3Li and belong to the cubic crystal system.² In the unit cell, the Li atoms are located at 000, while the Al atoms are located at 110, 101, and 01121. (a) Draw the unit cell of Al3Li. (b) Which of the crystal structures from Callister Chapter 3 does this resemble? Why do we not call it that structure? (c) Given that the lattice parameter is 0.401 nm, what is the density of Al3Li?arrow_forwardFigure 1 shows the Mg/Pb phase diagram. 700 L L 600 + M a + L Mg, Pb 500 465 400 L. Mg Pb 300 200 a + Mg Pb B+ Mg Pb 100 20 40 60 80 100 Mg Composition (wt% Pb) Pb Mg Pb Figure 1 The Mg/Pb phase diagram QUESTION 1-A Show on the diagram the following: 1. The Pb% to gain alpha grain with Mg.Pb on the boundary of alpha at the room temperature 2. Is it possible to gain beta grain with Mg.Pb on the boundary at the room temperature of an alloy? Explain 3. What is the mount of alpha in an alloy (50%Mg +50%Pb) at temperature of 500°C? 4. What is the mount of alpha in the alloy (50%Mg +50%Pb) at the room temperature? 5. What is Mg2Pb? Explain 6. If the requirement for an alloy to have 100 pph eutectic at the room temperature, what is the metal Pb percent? 7. Is it possible to have an alloy containing beta and alpha at the room temperature? Explain Temperature (°C)arrow_forwardCalculate the weight fraction for phases at point B? what are those phases? Is there any eutectic rection? How many phases are present in this diagram? Cu-Ni system T(°C) TA 1300 -L (liquid): A tie ļine liquidus L +a Тв EB solidus (solid) 1200 TD ID 20 3032 35: 4043 50 Ca wt% Niarrow_forward
- A NIO-20 mol % Mgo ceramic is allowed to solidify. (See the figure below.) Temperature (°C) 2800 2600 2400- 2200 2000 L (Ni, Mg)0 40 60 80 MgO Mole percent MgO NIO 20 (a) Determine the composition (in mol% MgO) of the first solid to form. 80 x (b) Determine the composition (in mol% MgO) of the last liquid to solidify under equilibrium conditions. 35 X%arrow_forwardCan you please solve this problem and show all of your workarrow_forwardHow would I... Determine the composition of aluminum and silicon (%Al/%Si) to melt the Al-Si solution at the lowest possible temperature. Please explainyour results...TIAarrow_forward
- For alloy of two hypothetical metals A and B, there exist an a, A-rich phase and a B, B-rich phase. For alloys of two different overall compositions at 25°C, the weight fractions of Wa and WB are listed in the table. Determine the solubility limits for both a and B phases at 25°C. Alloy Composition Wa WB 70wt% A-30wt% B 0.78 0.22 35wt% A-65wt% B 0.36 0.64arrow_forwardUsing the following table, draw a melting point diagram and estimate the eutectic temperature and composition: Percent composition Melting point range (°C) 100% A 125-126 75% A-25% B 115-120 65% A- 35% B 128-131 50% A- 50% B 135-140 100% B 151-152 Can you suggest what additional points you need to give a more accurate measure of the eutectic point?arrow_forwardConsider 1 kg of an Al-Si alloy with 20 wt % silicon. The sample is slowly cooled down from 900 oC to room temperature. The Al-Si phase diagram is given below. (a) What is the melting point of Al and Si? (b) Upon cooling, at what temperature will the first solid appear and what is the phase of the first solid, and what is the composition of the first solid? (c) At 600oC, what phases do you have and what is the mass of each phase? (d) At 578oC, what phases do you have and what is the mass of each phase?arrow_forward
- At what temperature does the first liquid phase form at 90% Ni? T(°C) 1600 1500 1400 1300 1200 1100 1000, L (liquid) L+α solidus liquidus 80 100 20 40 60 80 wt% Niarrow_forward700 L 600 м a + L Mg, Pb 500 O 465 400 Mg Pb 300 200 a + Mg Pb B+ Mg,Pb 100 20 40 60 80 100 Mg Composition (wt% Pb) Pb Mg Pb Figure 1 The Mg/Pb phase diagram 1. The Pb% to gain alpha grain with Mg2Pb on the boundary of alpha at the room temperature. ANSWER: From the diagram, the required Pb% is 40% since it corresponds to the boundary eutectic. 2. Is it possible to gain beta grain with Mg2Pb on the boundary at the room temperature of an alloy? Explain ANSWER: It is not possible to get grain on Mg2Pb since the eutectic composition will be varied, which in turn will lose the composition strength. 3. What is the mount of alpha in an alloy (50%Mg +50%Pb) at temperature of 500oC? ANSWER: The mount of alpha in the alloy 50% Mg + 50 % Pb at 500 C will correspond to the eutectic composition at the 70% of Pb, i.e; the mount = 70/100 = 0.7 % of Pb 4. What is the mount of alpha in the alloy (50%Mg +50%Pb) at the room temperature? ANSWER: The mount of alpha in the alloy 50% Mg + 50 % Pb at room…arrow_forwardA 42 wt% Pb-58 wt% Mg alloy is heated to a temperature within the α + liquid phase region shown in Animated Figure 9.20. If the composition of the α phase is 30 wt% Pb, determine (a) The temperature of the alloyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY