
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
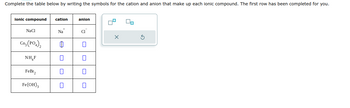
Transcribed Image Text:Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you.
ionic compound cation
NaCl
Co3(PO4)2
NH₂F
FeBr₂
Fe(OH)3
Na
0
anion
0
X
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name each of the following binary ionic compounds. a. AuCl b. KCl c. AgCl d. CuCl2arrow_forwardWrite formulas for the following compounds, all of which contain polyatomic ions. a. Aluminum nitrate b. Iron(III) sulfate c. Calcium cyanide d. Lead(IV) hydroxidearrow_forwardDistinguish between the following terms. a. molecule versus ion b. covalent bonding versus ionic bonding c. molecule versus compound d. anion versus cationarrow_forward
- (a) Write the formulas of all neutral ionic compounds that can be formed by combining the cations Na+ and Ba2+ with the anions S2 and PO43. (b) Iron forms ions having 2+ and 3+ changes. Write the formulas of the compounds formed between chloride ions and these two different iron cations.arrow_forwardGive the chemical symbol for each of the following ions. a. An oxygen atom that has gained two electrons b. A magnesium atom that has lost two electrons c. A fluorine atom that has gained one electron d. An aluminum atom that has lost three electronsarrow_forwardSulfur dioxide, SO2, is a molecular compound that contributes to acid rain, and CaCO3 is an ionic compound that can neutralize acid rain. Explain the difference in the meanings of these two formulas.arrow_forward
- For the following pairs of ions, use the principle of electrical neutrality to predict the formula of the binary compound that the ions are most likely to form. Na+andS2e.Cu2+andBr K+andClf.Al3+andI Ba2+andO2g.Al3+andO2 Mg2+andSe2h.Ca2+andN3arrow_forwardIn each box, write the chemical formula of the compound formed by the cation at the head of the column and the anion at the left of the row, and then write the name of the compound. Refer only to a clean periodic table when completing this exercise. Ions Na Hg2 NH4 Pb2 Mg2 Fe3 Cu2 OH- BrO- CO32- ClO32- HSO4- Br- PO43- IO4- S2-arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning