
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
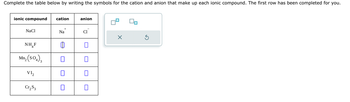
Transcribed Image Text:Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you.
cation
anion
ionic compound
NaCl
NH⭑F
Mn2(SO4)3
Cr₂S3
Na
☐
☐
☐
☐
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: some ionic compounds cation anion empirical formula name of compound 4+ Sn BrO 4 2+ Со NO, 4+ Pb cio 4 Po 2+arrow_forwardWrite the symbols for the carions and anions that make up each compoundarrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds cation anion empirical formula name of compound 2+ Fe F 3+ Fe F K F Sr 2+ Farrow_forward
- Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound cation + NaCl Na NH₂ Br 4 1 FePO4 CrF3 Cuo anion CI X Śarrow_forwardName these ionic compounds: MgS Al2O3 Ca(HSO3)2 Sr3(PO4)2 Fe(OH)2 Pb(CH3COO)2 (NH4)2HPO4arrow_forwardGive the formula for an ionic compound formed from each pair of ions. Na+ and O2− Mg2+ and S2- Al3+ and F− Ca2+ and P3−arrow_forward
- Complete the table below by writing the symbols for the cation and anion that make up each ionic compound.arrow_forwardComplete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound NaC1 CuF₂ VI3 VCL FeBr3 cation anion Na □□□□ C1 0 0arrow_forwardComplete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound cation NaCl Co3(PO4)2 NH₂F FeBr₂ Fe(OH)3 Na 0 anion 0 X Sarrow_forward
- Complete the table below by writing the symbols for the cation and anion that make up each ionic compound. The first row has been completed for you. ionic compound NaCl AuF FeI₂ CrBr₂ CuCl cation anion + Na 0 CI X Śarrow_forwardImagine two new elements have been discovered with symbols Q and Z. Q is in group 2A and Z is in group 7a on the periodic table. What is the formula of the ionic compound that these two elements can form? Q₂Z₂ QZ₂ Q₂Z QZ ZQ₂ O Z₂Q2 O Z₂Qarrow_forwardFill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds empirical formula name of compound cation anion 4+ Pb NO, 2+ Cu BrO4 2+ Mg co 2- CO3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY