
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
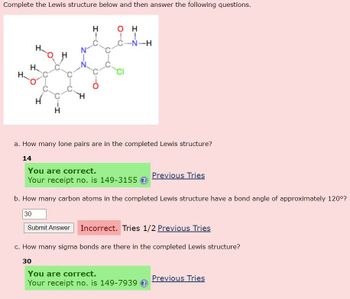
Transcribed Image Text:Complete the Lewis structure below and then answer the following questions.
нан
H
H
H
H
OH
C-N-H
a. How many lone pairs are in the completed Lewis structure?
14
You are correct.
Your receipt no. is 149-3155 Ⓒ
Previous Tries
b. How many carbon atoms in the completed Lewis structure have a bond angle of approximately 120°?
30
Submit Answer Incorrect. Tries 1/2 Previous Tries
c. How many sigma bonds are there in the completed Lewis structure?
30
You are correct.
Your receipt no. is 149-7939
Previous Tries
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Decide whether the proposed Lewis structure below is reasonable. proposed Lewis structure Is this a reasonable structure? H. Yes C=N- ONo O Yes ONo H. H. O Yes C=C=C ONo H. H. O Yes ONo H一arrow_forwardWhat is the working and solution to find the Carbon (marked with *) bond angle?arrow_forwardAbout t You dor esc L ! 1 compound Q A etches: the lines stand for chemical bonds between the atoms. Just ignore the dots. They stand for "lone pairs, and you'll learn about the ed to know anything about lone pairs to solve this problem. N A B с D Explanation 2 W S 28 command X H-O-H H-O-C- H :O: || H-C-C-Ö * sketch of molecules in it Check Н # 3 HO: H :O: H H. E D ī H -0-0-H 4-0- 20 -0- C Н H :0: C C $ 4 C :O: ö HÖC=C-Ö-H -C-0-H H R H :0: 0: F 888 :0: % 8 5 V HIGIA T G I -H A 6 group (Choose one) ▼ (Choose one) ▼ (Choose one) ▼ B (Choose one) ▼ MacBook Pro Y H & 7 4 U N © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | * 00 X ▶II 8 J - M ( 9 K O V K -0 O L 4 P command - A {arrow_forward
- O ELECTRONIC STRUCTURE AND CHEMICA... Deciding whether a Lewis... Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure [O=C-H]* :0: : CIC CI: [¤¤-6: 0/5 Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons but doesn't satisfy the octet rule. 000 The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: 0 Alia V No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* X Ar If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example,…arrow_forward3. Which two of the following structures represent correct Lewis structures of a compound with molecular formula C2H4O? H H H ö: H-c-c H :ö-H C=0=c н-с-о-с-н H C=C H-C=C-ö-H H H H H H H H 3 3 and 4 2 and 5 2 and 4 O 1 and 3 2.arrow_forward1.44 Draw one valid Lewis structure for each compound. Assume the atoms are arranged as drawn. H HCC NO H a. CH₂N₂ HCNN H H b. CH₂NO₂ HCNO HO C. CH3CNO d. (CH₂CN) HC CN H 1.45 Draw an acceptable Lewis structure from each condensed structure, such that all atoms have zero formal charge. a. diethyl ether, (CH3CH₂)2O, the first general anesthetic used in medical procedures b. acrylonitrile, CH₂CHCN, starting material used to manufacture synthetic Orlon fibers c. dihydroxyacetone, (HOCH₂)₂CO, an ingredient in sunless tanning products d. acetic anhydride, (CH3CO)2O, a reagent used to synthesize aspirinarrow_forward
- I answered most of it but it’s NOT GRADED. I need help with C,D and E.arrow_forwardSubject:arrow_forwarda.edu/d2l/Ims/quizzing/user/attempt/quiz_start_frame_auto.d2l?O%3D220518&isprv3&drc%3D1&qi=20426&cfql%3D0&dnb3D0 orcement nna Claiborne: Attempt 2 Write the Lewis structures of the indicated chemical substance and use it to answer the other questions. E. CO a) Draw the Lewis structure. b) Indicate which atoms in the species need what hybrid orbitals in order to explain the bonding in the molecule or ion. (Please write for example: sp4 hybrid orbitals on Na.) Use proper spelling or symbol for all elements. AV C) 1st type of bond: "There is a bond between the _-_ atom and the atom formed by the overlap of a orbital on the atom.) (Fill in the blanks from left to right). atom and the orbital on the triple bond C. a :arrow_forward
- Decide whether the Lewis structure proposed for each molecule is reasonable or not. proposed Lewis structure Is this a reasonable structure? If not, why not? molecule O Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. NH, H N=H The correct number is:| No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: LI Yes, it's a reasonable structure. :F: O No, the total number of valence electrons is wrong. BF, The correct number is: :F-B –F: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: U Yes, it's a reasonable structure. F No, the total number of valence electrons is wrong. The correct number is: IF No, some atoms have the wrong number of electrons around them. .. F: The symbols of the problem atoms are: U * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as…arrow_forwardQUESTION 3 What is the electronic configuration for the magnesium ion 1s2 2s2 2p63s2 01s2 2s2 2p6 e1s2 2s22p4 1s2 2s22p63s1 C152 2s2 2p63s22p QUESTION 4 Rank the indicated C-C bonds in increasing order of bond length.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY