
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please help me solve this problem, explain and make sure everything is correct 100% thanks
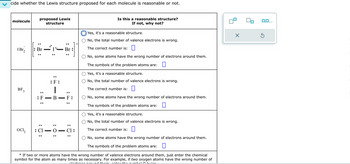
Transcribed Image Text:cide whether the Lewis structure proposed for each molecule is reasonable or not.
molecule
proposed Lewis
IBr₂
structure
Is this a reasonable structure?
If not, why not?
Yes, it's a reasonable structure.
No, the total number of valence electrons is wrong.
: Br
Br
The correct number is:
BF 3
:
.
:F:
|
: F B
::
: 0 :
ocl2
: Cl
- O
-
..
F:
::
Cl:
No, some atoms have the wrong number of electrons around them.
The symbols of the problem atoms are: ☐
Yes, it's a reasonable structure.
No, the total number of valence electrons is wrong.
The correct number is:
No, some atoms have the wrong number of electrons around them.
The symbols of the problem atoms are:
Yes, it's a reasonable structure.
No, the total number of valence electrons is wrong.
The correct number is:
No, some atoms have the wrong number of electrons around them.
The symbols of the problem atoms are:
*If two or more atoms have the wrong number of valence electrons around them, just enter the chemical
symbol for the atom as many times as necessary. For example, if two oxygen atoms have the wrong number of
0,0,...
ك
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- You were tasked to separate the components of a mixture containing silica, sodium chloride and charcoal. TNāCI dissolves in water while silica and charcoal are not water-soluble. Only charcoal dissolves in carbon disulfide. a. Write a short experimental procedure to carry out the separation of the mixture. b. Given the following data, determine the percentage of charcoal, sodium chloride and silica. Mass (g) Mass of beaker 100.000 Mass of beaker + mixture 110.000 mass of evaporating dish 62.000 mass of evaporating dish + solid after evaporation of water 65.000 Mass of beaker + charcoal + silica after evaporation of excess water 117.000 mass of beaker + silica after decanting dissolved charcoal and drying 113.545arrow_forward3. Standard white vinegar you can buy in the grocery store is 5% concentration. That means 5% of the liquid vinegar is acetic acid and 95% of the solution is water. In a hardware store, you can buy industrial strength vinegar, which is 30% concentration. This means that 30% of the vinegar is acetic acid, and the remaining 70% is water. Samuel does another experiment, this time with 5% vinegar and 30% vinegar. He sets up two science fair volcanoes (in no particular order), each with the same temperature, mass of baking soda and volume of vinegar. But one volcano uses 5% vinegar and the other volcano uses 30% vinegar. He measures the volume of gas production for the'first minute of each reaction, and he records the data below. Volcano # 1 Time Volume of gas Volume of gas produced (mL) vs. Time (s) for Volcano #1 (s) produced (mL) 60 50 10 20 30 25 38 40 46 30 • Volume of gas produced (ml) 40 50 20 50 52 e 10 60 53 20 40 60 80 Time (s) Volcano # 2 Volume of gas produced (mL) vs. Time (s)…arrow_forward1. Develop a detailed separation scheme for the separation and determination of the percent composition of your sample which will be a mixture of NaCl, NH.CL, and sand. You are expected to use the properties of the components listed below and some of the techniques listed in the table in the pre-lab queries (and used in Separations I). Keep in mind that any chemistry student should be able to pick up your scheme, understand it, and use it to complete the separation and calculation of % composition. Place the scheme on a separate sheet of paper. Component Solubility (@25°C) Melting Point Hardness sodium chloride, NaCl 35 g/100 mL water 801°C soft ammonium chloride, NH&Cl 37 g/100 mL water sublimes 350°C soft sand, SIQ2 insoluble 1600°C hard Example of compounds in a container. Naci NH.CI sio2 Exploring the Chemical World, PGCC, 2003 4 RESULTS Show all calculations with units in this space.arrow_forward
- Part C: Determination of the alcohol content of unknown liquor St tiend Volume (mL) Sample ТИШТИНА 0.00% alcohol 10.0% alcohol 20.0% alcohol 40.0% alcohol 50.0% alcohol Unknown Mass (g) 1o podina ko 9.8919 9.7899 9.600 9 9.420 д % Alcohol content of the liquor_ 9.2155 9.5089 2VLELA CVOLION EAEK EVI OK DRA IVBOKY LOBAN ocen 10.00 10.00 10.00 10.00 10.00 10.00 Density (g/mL) 0.00% alcohol calculation: ися → о 0.99 во 0.99 Ino7 sm dolaW 120.00 supinubst sdi na LANG 411 be: [scordas: 0.97 0.94 in oil to notisalarisi 0.92 0.95arrow_forwardSHOW COMPLETE SOLUTION AND BOX THE FINAL ANSWER.arrow_forwardPart C: Determination of the alcohol content of unknown liquor: Volume (mL) Sample ТИШТИНА 0.00% alcohol 10.0% alcohol 20.0% alcohol 40.0% alcohol 50.0% alcohol Unknown Mass (g) 1o podina ko 9.8919 9.7899 9.600 9 9.420 д % Alcohol content of the liquor 9.2155 9.5089 2VLELA CVOLION EAEK EVI OK DRA IVBOKY LOBAN ocen 10.00 10.00 10.00 10.00 10.00 10.00 tiend Density (g/mL) 0.00% alcohol calculation: ися → о 0.99 во 0.99 Ino7 sm dole 120.00 supinubst sdi na LANG 411 be: [scordas: 0.97 0.94 in oil to notisalarisi 0.92 0.95arrow_forward
- 7. You can see an MSDS below. Please answer the following questions related to the MSDS. a) What is the name of this chemical? b) What should you do if someone drinks the chemical? c) Would this chemical catch on fire if it was exposed to flames? d) If this chemical gets in your eye what should you do? e) What color is this chemical? f) What should you do if someone spills a small amount of the chemical?arrow_forwardPlease give information about these chemicals.arrow_forwardUnit 3-Two slides 11. Create a question that involves your compound and the formula m= n.M. Solve the question and show all of your work. wwwww 12. Create a question that involves your compound and the formula n = N/NA. Solve the question and show all of your work. TEarrow_forward
- он ㅅ ·Br OH Multistep synthesisarrow_forwardA student prepares a 1.1 mM aqueous solution of 4-chlorobutanoic acid (C3H, CICO,H). Calculate the fraction of 4-chlorobutanoic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. % X Sarrow_forwardPls help ASAP on all PLS I REQUESTarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY