
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
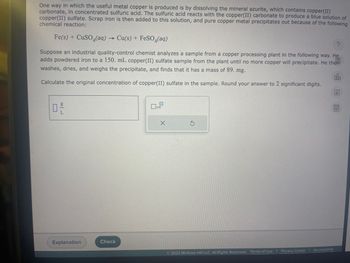
Transcribed Image Text:One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II)
carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of
copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following
chemical reaction:
Fe(s) + CuSO (aq) → Cu(s) + FeSO4(aq)
?
Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He
adds powdered iron to a 150. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then
washes, dries, and weighs the precipitate, and finds that it has a mass of 89. mg.
00.
Calculate the original concentration of copper(II) sulfate in the sample. Round your answer to 2 significant digits.
0
|| 09
Explanation
Check
x10
X
G
2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Classify each of the following reaction as: precipitation, acid-base or oxidation-reduction (red-ox). Chemical reaction Classification C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(g) Lead(II) acetate(aq) + Sodium sulphate(aq) → Lead(II) sulphate(s) + Sodium acetate(aq) Copper(II) chloride(aq) + Zinc metal → Cu(s) + ZnCl2(aq) H2SO4 (aq) + 2 NaOH (aq) → Na2SO4 (aq) + 2 H2O (l)arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe (s) + CuSO4 (aq) → Cu (s) + FeSO4 (aq)Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 350.mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 73.mg . Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits. gLarrow_forwardA 4.50 g sample of limestone (impure CaCO3) was dissolved in 0.1M HCl solution according to the following equation. CaCO3(s) + 2HCl(aq) → CaCl2 (aq) + CO2 (g) + H2O (l) Excess of (NH4)2C2O4(aq), was added to the resulting solution to precipitate the calcium ions as calcium oxalate, CaC2O4(s). Ca2+(aq) + C2O42-(aq) → CaC2O4 (s) The precipitate was filtered, dried and weighed at 2.15 g. (Show your work) a) Determine the percentage by mass of calcium carbonate (CaCO3) in the limestone sample. (MM of CaCO3 is 100.09 g/mol) b) Why we need to dry the precipitate until we get a constant mass? c) Is the precipitate of calcium oxalate (CaC2O4) stable and in the final form or not? Explain.arrow_forward
- A chemistry student needs to standardize a fresh solution of sodium hydroxide. He carefully weighs out 10.mg of oxalic acid H2C2O4 , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250.mL of distilled water. The student then titrates the oxalic acid solution with his sodium hydroxide solution. When the titration reaches the equivalence point, the student finds he has used 12.2mL of sodium hydroxide solution. Calculate the molarity of the student's sodium hydroxide solution. Be sure your answer has the correct number of significant digits.arrow_forwardA chemistry student needs to standardize a fresh solution of sodium hydroxide. He carefully weighs out 50.mg of oxalic acid H2C2O4 , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250.mL of distilled water. The student then titrates the oxalic acid solution with his sodium hydroxide solution. When the titration reaches the equivalence point, the student finds he has used 26.2mL of sodium hydroxide solution. Calculate the molarity of the student's sodium hydroxide solution. Be sure your answer has the correct number of significant digits.arrow_forwardThe following reaction occurs when two aqueous solutions are mixed: Cr(NO3)3(aq) + 3NaOH(aq) → Cr(OH)3(s) + 2NaNO3(aq) Identify the spectator ion OR ions in the solution. SELECT ALL SPECTATOR IONS!arrow_forward
- To determine , by gravimetric analysis, the concentration of barium ions (Ba2+) in a given solution, 25.00cm3 of it are pipetted into a beaker and an excess of dilute sulphuric acid is added to it. The precipitate then obtained (BaSO4) is filtered, dried and weighed. The mass of the precipitate is found to be 1.167g Calculate the concentration of barium ions in the solution? (only 2 decimal places). _________Mol/Larrow_forwardTo measure the amount of citric acid (C₂H₂O(CO₂H)³) in a certain candy, an analytical chemist dissolves a 23.00 g sample of the candy in 250. mL of water and titrates this solution to the endpoint with 23.7 mL of 0.400 M sodium hydroxide (NaOH) solution. The balanced chemical equation for the reaction is: C_HO(CO,H),(aq) + 3OH (aq) → CHO(CO,) (aq) + 3H,O() What kind of reaction is this? If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of C3H₂O(CO₂H), in the sample. Be sure your answer has the correct number of significant digits. O precipitation O acid-base O redox 0 0 % 뮤 X 8 ☐arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe(s) + CuSO4(aq) → Cu(s) + FeSO4(aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 200. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 107. mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits. 0 x10 х §arrow_forward
- The concentration of SO42– ions in a 45.0 mL sample of seawater is determined by adding a solution of BaCl2 and precipitating the SO42– as BaSO4. After the precipitate is filtered from the solution, it is dried and weighed. If the mass of BaSO4 recovered is 0.315 g, what is the sulfate concentration of the seawater sample? Express your answer in mmol/L.arrow_forwardTartaric acid, H2C4H4O6, is a diprotic acid that naturally occurs in the production of wine. Jose was tasked to find out the acid content in a 100.0 mL wine sample by titrating it with a standardized solution of NaOH. He was able to establish the working concentration of the NaOH solution by using 35.21 mL of it to titrate a primary standard of KHP that weighed 3.001 g. The grams and %w/w of Tartaric acid present in the 100.0 mL wine sample were then determined by titrating a 25.00 mL aliquot using 43.56 mL of the standardized NaOH solution. H2C4H4O6 (aq) + 2NaOH (aq) ↔ Na2C4H4O6 (aq) + 2H2O (l) Assume that the density of the wine sample is 1.000 g/mL MM of KHP = 204.22 g/mol MM of H2C4H4O6 = 150.087 g/mol What is the molar concentration of the prepared NaOH solution?…arrow_forwardThe following reaction is a precipitation reaction. NaCl (aq) + AgNO3 (aq) → NaNO3 (aq) + AgCl (s) a metal displacement a decomposition an acid-base neutralizationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY