
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Chrome
File
Edit
View
History Bookmarks People Tab
Window
Help
80%
Tue 8:42 PM
Chem 101 HW
101 Chem101
+
app.101edu.co
E
Question 22 of 25
Submit
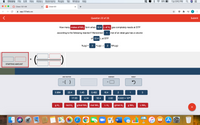
How many moles of NH3 form when 32.4L of H2 gas completely reacts at STP
according to the following reaction? Remember
1 mol of an ideal gas has a volume
of 22.4 L at STP
N2(g) +
3
H2(g) →
2
NH3(g)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
0.964
22.4
1.45
0.482
16.4
2
1
17.03
4.34
32.4
2.02
6.022 x 1023
g H2
mol H2
g/mol NH3
mol NH3
LH2
g/mol H2
g NH3
L NH3
PAGES
W
+
Expert Solution
arrow_forward
Step 1
Given synthesis reaction of NH3 (g) is written as follows;
N2 (g) + 3 H2 (g) ----> 2 NH3 (g)
given volume of H2 (g) = 32.4 L
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9-please fastarrow_forwardRemaining Time: 1 hour, 01 minute, 47 seconds. * Question Completion Status: QUESTION 2 The number of water molecules in 2.3 mg of water is O 6.02 x 1023 O 77.0 x 1023 O 7.7 x 1023 O 7.7 x 1019 none of the above QUESTION 3 A ctudent makes a colution bu discolvina 254a ofNDOH int0 450 gof water Wh. Click Save and Submit to save and submit. Click Save All Answers to save all answer ere to search DELLarrow_forwardQuestion 16 of 96 Submit Determine the number of atoms of O in 54.1 moles of Cr3(PO4)2. | atoms 1 2 4 6. C 7 +/- x 10 0 Tap here or pull up for additional resources LO 00arrow_forward
- A Login My AP Login - Coll. LanguageTool -Onl.. Co Biography of Albert. Crillegnettouth Pre-AP Unit 3 Learning Checkpolnt 2 1 (3 10 11 Question 9 D CH, (g) + 2 O2 (g) CO, (g) + 2 H20 (g) When CH, (g) is burned in O,(g), the reaction represented by the equation occurs. If 32 g of CH, is burned completely, how many moles of CO, are produced? Enter the number of moles to the nearest whole number. molarrow_forwardQuestion 11 of 29> < The reaction for producing glucose in plants, called photosynthesis, is light 6 CO2 + 6 H20-CH1206+6 O2 If a plant produces 2.41 mol CHOhow many moles of H,O are needed? 65 12 moles H,O: mol help terms of use contact us privesy policyarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY