
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please calculate percent yield

Transcribed Image Text:Can you find out why?
Procedure
To a 50 mL round-bottomed flask equipped with a stir bar add paraformaldehyde
(0.30 g) and 95% formic acid (2 mL). Equip a condenser, stir the mixture, and heat
gently using a heating mantle. After boiling for 5 min, add mesitylene (4 mL) from
the top of the condenser. Reflux the mixture for 2 hours.
Remove the heat and let the reaction cool to room temperature. Place the flask in an
ice bath to promote crystal formation. Vacuum filter the crystals, you may need to
use a spatula to break the big chunks. Rinse the flask with water (2 x 5 mL) to
transfer all the solids. Wash the solids with sodium bicarbonate solution (5 mL) and
then again with water (5 mL). Lastly, wash the crystals with ice-cold methanol (5
mL) to remove the unreacted mesitylene. Allow the solids to dry on your bench for
15 min. Obtain the mass and the melting point.
bloid al
Using a separatory funnel, separate the organic and aqueous layers and discard each
in the appropriate waste container.
Dimesitylmethane is a symmetric molecule and will give a simple and rather
uninteresting 1H NMR. Your instructor will decide if you need to run an NMR or
perform a civic engagement assignment.
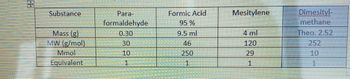
Transcribed Image Text:Substance
Para-
formaldehyde
Formic Acid
95%
Mesitylene
Mass (g)
MW (g/mol)
Mmol
0.30
9.5 ml
4 ml
Dimesityl-
methane
Theo. 2.52
30
46
120
252
10
250
29
10
Equivalent
1
1
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps

Knowledge Booster
Similar questions
- Design a solution stoichiometry problem that involves a limiting reagent and percent yield. Include a chemical reaction. White question in a sentence. And show the solution.arrow_forwardMULIIPLE CHUICE QUESTION Which one can you NOT use to compare amounts in a chemical reaction? mass moles molecules Rewatch Subrt !!arrow_forwardCalculate limiting reagent and percent yieldarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY