
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
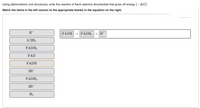
Transcribed Image Text:Using abbreviations (not structures), write the reaction of flavin adenine dinucleotide that gives off energy (-AG).
Match the items in the left column to the appropriate blanks in the equation on the right.
H+
FADH → FADH3 + H+
3/2H2
FADH3
FAD
FADH
2H+
FADH2
3H+
H2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- In addition to the reactions mentioned in Section 23.5, PLP can catalyze b-substitution reactions. Propose a mechanism for the following PLP-catalyzedb-substitution reaction:arrow_forwardMany enzymes are switched "on" by attachment of a phosphate group at a specific serine somewhere on the protein (phosphorylation). The basic reaction is: E + ATP2 Ep + ADP Po SERINE PHOSPHO SERINC (Note the "squiggles" before the backone amide and carbonyl indicate the polypeptide chain continues on either side of the serine). For phosphorylation to have this effect, there has to be some equilibrium between inactive and active forms conformations of the enzyme: [Eactive] [Einactive] Einactive 2 Eactive; K* The same basic equilibrium must exist for the phosphorylated protein: [Ep,active] [Ep,inactive] EP,inactive 2 Ep,active; Kp = (a) If phosphorylation increases the measured activity of the enzyme, is K* or K larger? Why? (b) Does the phosphorylation site need to be near the site where the enzyme binds its substrate (e.g. the reactant whose chemistry it catalyzes)? Why or why not?arrow_forwardThe following are structural diagrams of a selection of newly discovered amino acids. OH -の-CHs NH HO C-OH NH, AN-CH CH2 CH2 OH Ho NH, C=0 a) Select 1 amino acid. Redraw it. Label the alpha carbon and circle/highlight the entire backbone of the amino acid. b) The amino acids are part of a channel protein embedded in the cell membrane. Choose 2 amino acids (from above) that you would expect to find within the interior/middle of the cell membrane. Draw the formation of the dipeptide using the 2 amino acids you selected. Identify the other products formed in the reaction.arrow_forward
- Draw a schematic of the chemical structure of the Leu-Tyr-Met-Lys tetrapeptide, with the correct ionization at neutral pH (pH 7). Also, give an estimate the pl of this tetrapeptide (you are given that the pKa of any acidic R group is 4, the pKa of any basic R group is 11.5, and the pKa of the α-COOH is 2, and the a-NH3 is 9.5). Provide rationale!arrow_forwardi subimtted this question on other websites and they were wrong, please dont copy pastearrow_forwardTitration of alanine by a strong base, for example NaOH, reveals two pk's. Which is the titration reaction occurring at pK1 (pK1 = %3D 2.34)? O-NH2 + COH →-NH + H2O O-COOH + OH →-CO + H20 -COOH + -NH2 → -COO" + -NH2+ -CoO +-NH2* → -COOH +-NH2 O -NH3* + OH →-NH2 + H2Oarrow_forward
- Write a DNA sequence that would encode the amino acid sequence valyl-tryptophanyl-lysyl-prolyl-phenylalanyl-threonine.arrow_forwardExplain the one turn/two turn joining (12/23; heptamer/nonamer) rule for VL to JL, DH to JH, and VH to DHJH joining.arrow_forwardThe commercial sample of carboxyl esterase contained 15.0 mg of enxyme in 0.30 mL of suspension. In your experiment, you add 0.090 mL of this suspension to the sample tube that contains a total volume of 3.0 mL. How many mg of carboxyl esterase enzyme do you have in the sample tube?arrow_forward
- Answer the following using the structure: a. Is this a purine or pyrimidine? b. Is this a deoxyribonucleotide or a ribonucleotide? c. Indicate the component that changes this from a nucleoside to a nucleotide.arrow_forward1/v 1/[S] Compound I is a substrate for the enzyme reverse transcriptase and compounds Il and lll are inhibitors. The inhibition plots for each compound are Illustrated above. The line labeled E is the enzyme with substrate alone (no inhibitor present). Answer the following questions by filling în the blanks. Line A most likely belongs to compound because it is a inhibitor. Line B belongs to compound because it is a inhibitor.arrow_forwardDraw the peptide formed between asparagine and histidine. H,N- -CH-C-OH H,N-CH-ċ-OH ČH2 ČH, N° NH2 -NH +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON