
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
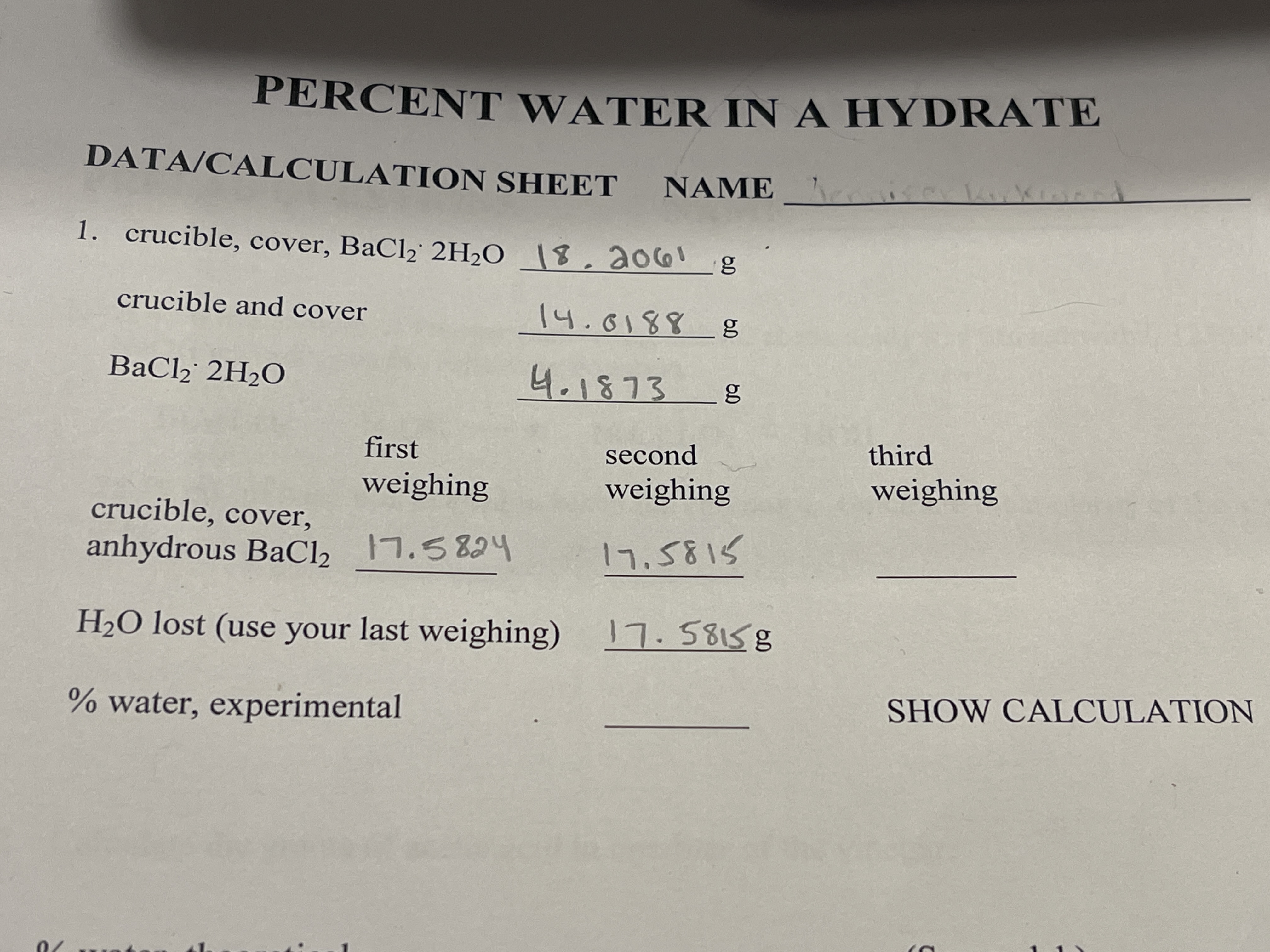
Transcribed Image Text:PERCENT WATER IN A HYDRATE
DATA/CALCULATION SHEET NAME
1. crucible, cover, BaCl2 2H2O \8,ao6!
crucible and cover
14.0188 g
BaCl, 2H2O
4.1873
first
second
third
weighing
weighing
weighing
crucible, cover,
anhydrous BaCl, 7.5824
17.5815
H2O lost (use your last weighing) 7.58158
% water, experimental
SHOW CALCULATION
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Data Trial 1 Trial 2 Mass of empty crucible with lid 26.698g 26.687g Mass of Mg metal, crucible, and lid 27.060g 27.046g Mass of MgO, crucible, and lid 27.291g 27.273g Determine the average percent yield of MgO for the two trials. ( I average the percent yield of the two trials) 98.55% (Just write out how you got it)arrow_forwardI keep trying to solve but I am still unsurearrow_forwardAnswer this question in the same format thats in the picture below on paper, answer correctly.Question: how many grams are in 1.73 mol of dinitrogen pentoxide (N2O5)arrow_forward
- 5.71 g of nitrogen reacts with a certain amount of hydrogen according to the following reaction: 3 H2 + N2 → 2 NH3 How many grams of hydrogen would be required to react with 5.71 g of nitrogen ? Select ALL the conversion factors needed for this calculation using dimensional analysis method. 1) 1 mol N2/3mol H2 2) 28.02 g N2/ 1mol N2 3)1 mol H2/2.016 g H2 4)2.016 g H2/1mol H2 5)1 mol N2/28.02 g N2 6) 3 mol H21 mol N2arrow_forwardHow would i solvearrow_forwardmistry app.101edu.co YouTube Maps NG AMOUNT * @ 7 2 X W S F2 X Jill # Welcome to MyTCC Welcome to MyTCC 3 F3 E D C 20: $ 4 R F 30 % 35.6 V Aluminium has a density of 2.70 g/cm² How many moles of aluminum are in a 13.2 cm block of the metal substance? 4 ADD FACTOR X x() F5 5 molecules Al T Content G 1.32 F6 A 6 B 2.70 0.489 g Al/mol F7 Y & H Question 1 of 50 0.100 g/cm² W 7 26.98 N ASUS X F8 U J g Al Lo b Home | bartleby ANSWER 8 962 13.2 F9 | M mol Al 1 1 K F10 9 < I RESET 5 6.022 x 10 O cm 1 F11 O L X + 10 P : F12 - I Prt Sc 1 ? + [ Q 12 ☆ = 12 Insert 1 Delete Backspace J Submit Home Enter PgUp Shift Pearrow_forward
- Esc STARTING AMOUNT 71°F Sunny Z²² F1 DX X F2 A- F3 F4 According to the balanced reaction below, calculate the quantity of moles of NH, gas that form when 4.20 mol of N₂H4 liquid completely reacts: 4.20 5.60 ▬▬ *- ADD FACTOR x( ) F5 17.04 1.40 g N₂ *+ O Search the web F6 3.15 3.37 x 1024 mol N₂H₁ 3 N₂H.(1)→ 4 NH.(g) + N2(g) ► / || F7 Question 9 of 11 2.53 x 1024 32.06 4 g NH, F8 12.6 g N₂H4 28.02 ANSWER 1.05 4 pity mol NH, F9 1 6.022 x 1023 8.43 x 102 3 * RESET mol N₂ 2 16.8 F10 Insert F11 F12 Deletearrow_forwardCan you please solve using box method?arrow_forwardEmpirical Formula for Unknown Sample Data1. Mass of crucible 28.072(grams)2. Mass of crucible + Unknown 30.528(grams)3. Mass of crucible + Na + S 29.782(grams)4. Mass of crucible + Na 28.786(grams)5. Mass of Na 0.714 6. Mass of S 0.996 7. Mass of O 0.7468. Mass of Unknown Sample 2.51 using the information above find the moles for the following elements: Moles of Na Moles of S Moles of Oarrow_forward
- Conc. of NaOH: TRIAL 1 TRIAL 2 TRIAL 3 Mass of flask 81.061 69.692 96.039 Mass of flask and vinegar 83.010 71.578 97.940 Mass of vinegar 1.949 1.886 1.901 Volume of vinegar 2.00 2.00 2.00 Initial buret reading Final buret reading 17.0 17.5 16.0arrow_forward3 Answer in General Chemistry fo X A app.101edu.co ps M Gmail YouTube Maps Tradůzir GE Notícias Question 12 of 15 What mass in grams of NaN3 is required to produce 50.2 L of N2 gas (density = 1.25 g/L ) according to the balanced chemical reaction: 2 NaN3(s) → 2 Na(s) + 3 N2(g) STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) %3D 2 3 97.1 77.7 22.99 1 1.25 6.022 x 1023 65.02 50.2 28.02 146 218 g N2/L mol N2 mol Na mol NaN3 g N2 g NaN3 g Na L N2 山T 286 APR 1 PAGES W 17 MacBook Air ....arrow_forwardData Trial 1 Trial 2 Mass of empty crucible with lid 26.698g 26.687g Mass of Mg metal, crucible, and lid 27.060g 27.046g Mass of MgO, crucible, and lid 27.291g 27.273g Determine the percent yield of MgO for your experiment for each trial. Trial 1:98.8% Trial 2:98.3% (Just write out how you got both percent yields and follow signifigant figure rules)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY