
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Blackboard Learn X
← → C
M Gmail
VO
<
app.101edu.co
Aktiv Chemistry
STARTING AMOUNT
YouTube Maps: Welcome to MyTCC
X
S
X
X
E
b Search results for 'Det X
403- Forbidden: Ac...
ADD FACTOR
g CO₂
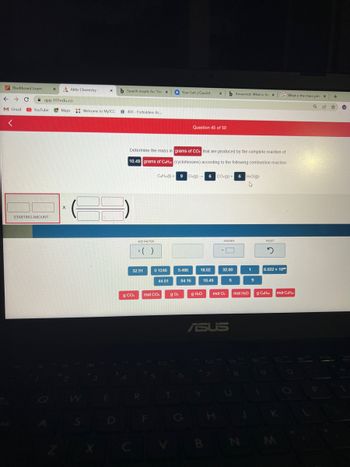
Determine the mass in grams of CO: that are produced by the complete reaction of
10.49 grams of CaHua (cyclohexane) according to the following combustion reaction:
* ( )
32.91
R
F
0.1246
CeHz(1) 9 O:(g) 6 CO(g) + 6H₂O(g)
+
4
44.01
mol CO₂
Your Sets | Quizlet X b Answered: What is the X
g 0₂
T
5.486
Question 45 of 50
84.16
18.02
g H₂O
10.49
ANSWER
Y
32.00
mol O
ASUS
6
U
1
mol H₂O
9
RESET
2
G What is the mass per X
Q 14
6.022 x 10
g CaHuz
M
mol CH
+
☆
W
Expert Solution
arrow_forward
Step 1
In the given question we have to calculate the gram of CO2 produces by the complete reaction of cyclohexane,
First of all, we have to calculate the moles of cyclohexane to react with oxygen then using mole-Ratio to find out the moles of CO2 then multiplying the moles of CO2 with molar mass of CO2 to get the mass of carbon dioxide.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Each step in the following process has a yield of 80.0%.CH4+4Cl2⟶CCl4+4HClCCl4+2HF⟶CCl2F2+2HClThe CCl4 formed in the first step is used as a reactant in the second step. If 2.00 mol CH4 reacts, what is the total amount of HCl produced? Assume that Cl2 and HF are present in excess.arrow_forwardNitric Oxide can be made from the oxidation of ammonia as shown below. A reaction between 8.5 g of NH3 and 25.0 g of O₂ produces 12.0 g of NO. What is the percent yield for this reaction? 4 NH3(g) + 5O₂(g) → 4 NO(g) + 6 H₂O(g) Molar masses (in g mol-¹): NH3 17.034, 0₂ 32.00, NO 30.01, H₂O 18.016arrow_forwardChlorine gas can be produced in the laboratory by adding concentrated hydrochloric acid, HCl, to manganese(IV) oxide according to the following reaction: MnO2 (s) + 4 HCl (aq) → MnCl2 (aq) + 2 H2O (l) + Cl2 (g) What mass of MnO2 is required to produce 48.4 g Cl2 by the following reaction? Be sure to enter a unit with your answer.arrow_forward
- Consider the following fictitious reaction. If the reaction begins with 0.143 mol of A and the actual yield of C is 13.16 g, what is the percentage yield? The molar mass of C is 144.63 g/mol. 4 A (g) + B (g) --> 3 C (g) 80.6 % 84.8 % 70.3 % 96.6 % 63.6 %arrow_forwardA sample of calcium metal with a mass of 2.00 was reacted with excess oxygen. The following equation represents the reaction that took place: 2Ca(s)+O2(g)-> 2CaO(s) the isolated product CaO weighed 2.26g. What was the percent yield of the reaction?arrow_forwardDetermine the mass in grams of CO₂ that are produced by the complete reaction of 0.05090 moles of C₇H₈ (toluene) according to the following combustion reaction: C₇H₈(l)+ 9 O₂(g) → 7 CO₂(g) + 4 H₂O(g)arrow_forward
- What is the limiting reagent when 10.0 g of carbon monoxide (CO, molar mass 28.01 g/mol) and 15.0 g of iron(III) oxide (Fe2O3, molar mass 159.7 g/mol) are allowed to react completely to form carbon dioxide (CO2) and iron (Fe) 3CO(g) + Fe2O3(s) --> 3CO2(g) + 2Fe(s) a)Fe2O3 b)CO c)CO2 d)Fearrow_forwardMorphine (C₁₇H₁₉NO₃) is a painkiller in the opiate family. A sample of morphine was discovered that had been diluted by mixing with table salt (sodium chloride). When 2.00 g of the mixture undergoes combustion, 3.17 g of CO₂ is produced. What is the mass percent of morphine in the mixture?arrow_forwardGlucose, C6H1206, can be represented by the molecular model shown below. If 1.00 mol of glucoseis submitted to combustion analysis, how many moles of CO2 and how many moles of H2O wouldbe formed?arrow_forward
- Morphine (C₁₇H₁₉NO₃) is a painkiller in the opiate family. A sample of morphine was discovered that had been diluted by mixing with table salt (sodium chloride). When 2.00 g of the mixture undergoes combustion, 3.97 g of CO₂ is produced. What is the mass percent of morphine in the mixture?arrow_forwardEthanol (C₂H₆O) is combusted in air according to the following reaction: C₂H₆O(l) + O₂(g) → CO₂(g) + H₂O(l) How many grams of water would be produced by the complete combustion of 6.45 moles of ethanol in the presence of excess oxygen?arrow_forwardWhat is the mass in grams of CO2 that can be produced from the combustion of 5.26 moles of butane according to the given equation: 2C4H10(g) + 13 O2(g) -> 8CO2(g) + 10H2O(g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY