
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Part H
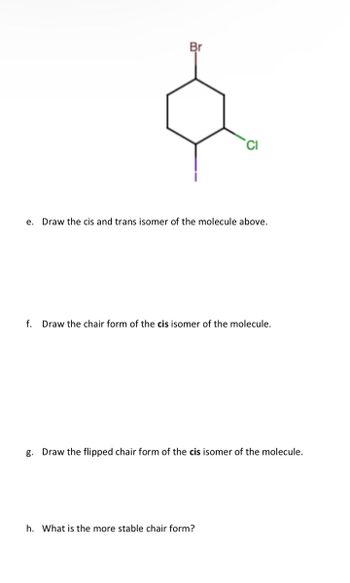
Transcribed Image Text:Br
e. Draw the cis and trans isomer of the molecule above.
f. Draw the chair form of the cis isomer of the molecule.
g. Draw the flipped chair form of the cis isomer of the molecule.
h. What is the more stable chair form?
Expert Solution
arrow_forward
Step 1: Explain cis-trans concept
The cis and trans isomers are called geometric isomers.
If both the substituents are present on same side then it is called a cis isomer.
If both the substituents are present on opposite side then it is called a trans isomer.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Emerson M Cremistr pdated late workE a educate.lindsay.k12.ca.us/iFrame.aspx?iCtri=STUDENT BASE HOME CONTROL O Empower 2021 O Ermpower 2021 pokmarks DE IXL Classes O Google Slides O Empower 2020 A Google Docs t Lindsay High School Physics Safety & Ski. E Guided pract 2-1 googlegalaxysclence.com Li Be 1-0 1-5 2-0 Al 2.5 3-0 3-5 Na Mg 1-2 Si S CI 0-9 1-5 1-8 Ge 2-1 2-5 3-0 K Ca Ga 1-6 As Se Br 0-8 1-0 1-8 2-0 2-4 Te 2-8 Rb Sr In Sn Sb 0-8 1-0 1-7 1-8 Pb 1-9 2-1 2-5 Cs 0-7 Ba TI Bi Po At 2-2 Review the chart above. It is showing how much energy it takes to remove an electron from each of those types of atoms. Why would 0.9 1-8 1-9 1-9 2-0 it be so high in the top right comer and so low in the lower len comer? It would be high in the top right coner because these electrons are closer to the nucleus and held more strongly, so they will take O A. more energy to remove. And the opposite is true for the lower left, farther from the nucleus and so not held as strongly, requiring less energy to…arrow_forwardData Sheet UNKNOWN NUMBER: 1 49.83 4575 8.08 12.47 I1.031 1.44 1. Mass of test tube + naphthalene 2. Mass of empty test tube %3D 3. Mass of naphthalene 4. Mass of vial + Unknown %3D 5. Mass of vial %3D 6. Mass of Unknown Cooling curve data Pure Naphthalene Naphthalene + Unknown Temp Time Temp Time Temp Time Temp Time 79 0.5 T4-9 5 20:7 5:30 78:2 020 749 S:30 85.2 30 84.5 B15 1:30 70.9 6 6.70 72 30 14.9 6 14.9 6:30 75.6 1:08C 74 9 75:52 20.0 69.8 7. 69.4 7:30 68.9 68:3 76:5. Imit 74 75.02.9 749,3. 28.2 2 76 Y 25.0 22. 3. . 2:10 "NS 130 3:30 24.0 9 23-4 9:30 74.9.4.30 23.5 8:)0 679 9 67.2 9.30 . 21.8 71.4 4 330 74.9 94.9 u १:१० :30 67.2 10 20.9 S b6.5 10.30 75-5 750 10:50 66.0 1 7. Freezing point of pure Naphthalene from cooling curve = 8. Freezing point of solution 9. AT 10. Molality of solution, m 11. Molar mass of Unknown 12. Include graphs of cooling curves CALCULATIONSarrow_forwardWhich conversion factors can be used to convert 1 teragram to kilograms showing proper dimensional anlysis ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY