
Appl Of Ms Excel In Analytical Chemistry
2nd Edition
ISBN: 9781285686691
Author: Crouch
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Data Sheet
UNKNOWN NUMBER:
1
49.83
4575
8.08
12.47
I1.031
1.44
1. Mass of test tube + naphthalene
2. Mass of empty test tube
%3D
3. Mass of naphthalene
4. Mass of vial + Unknown
%3D
5. Mass of vial
%3D
6. Mass of Unknown
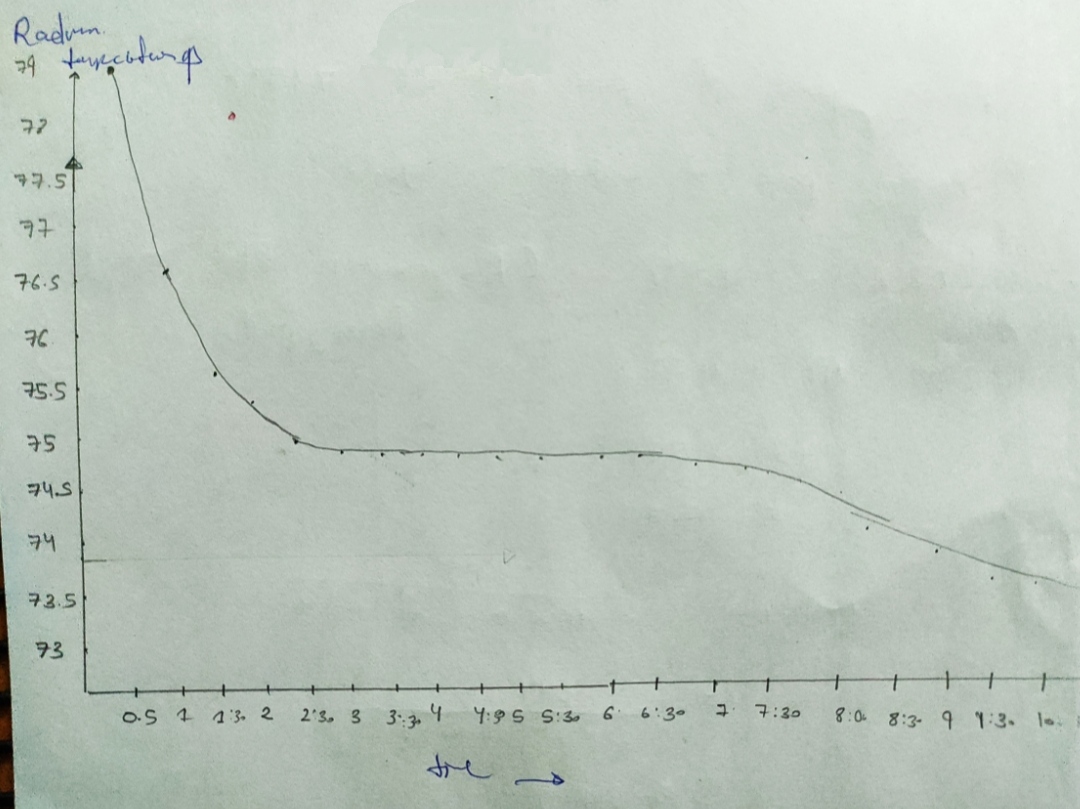
Cooling curve data
Pure Naphthalene
Naphthalene + Unknown
Temp
Time
Temp
Time
Temp
Time
Temp
Time
79 0.5
T4-9 5
20:7 5:30
78:2 020
749 S:30
85.2 30
84.5
B15 1:30
70.9 6
6.70
72 30
14.9 6
14.9 6:30
75.6 1:08C 74 9
75:52
20.0
69.8 7.
69.4 7:30
68.9
68:3
76:5. Imit
74
75.02.9
749,3.
28.2 2
76 Y
25.0
22. 3.
.
2:10
"NS 130
3:30 24.0 9
23-4 9:30
74.9.4.30 23.5
8:)0
679 9
67.2 9.30
.
21.8
71.4 4
330
74.9
94.9 u
१:१०
:30 67.2 10
20.9 S
b6.5 10.30
75-5
750
10:50
66.0 1
7. Freezing point of pure Naphthalene from cooling curve =
8. Freezing point of solution
9. AT
10. Molality of solution, m
11. Molar mass of Unknown
12. Include graphs of cooling curves
CALCULATIONS
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Doard.learn.xythos.prod/579872cf81db6/2987817?X-Blackboard-53-Bucket-blackboard.learn.xythos.prod&X-Blackboard-Expiration= S 1/2 CHEM1407 HF HBr #1) Identify if the following substances are a weak acid, strong acid or neither and write the dissociation equation for the acids LiOH H₂SO3 NH3 HC₂H302 HCIO4 HCIO HF H₂SO4 #2) Identify the following as a monoprotic, diprotic, or triprotic acids H3PO4 HC1O2 HNO3 100% + H₂C₂O4 Homework Ch 16, Sec 6A H Name: 63°F Mostly cloudyarrow_forwardM Mrs. X Asse X I Thor x A Clas x E cm r x 4 Engl x I Thor x W Dem x E Thoi x A Clas x Test x tao TẠO X A OnC x b oncourseconnect.com/assessment/1685550/1490c638-e828-11a5-deeb-89c8c289a008?testingcenter%=Dtrue&mobile%3false E TPSS Bookmarks Thomas Williams . CHEMISTRY (2021) ASSESSMENT CHEMISTRY I 12-3 (THOMAS WILLIAMS, ID: 12390719) Carbon-14 (C-14) is a radioactive isotope with a half-life of 5,730 years. It decays to stable nitrogen-14 (N-14) through beta decay. Which process is a current use of C-14? A reducing the size of certain tumors generating radiation for medical imaging C producing energy in nuclear power plants determining the age of buried organic materials Save/Exit 1 3 4 6.arrow_forwardMon Apr Content X b Answered: How many mL of X Bb SolutionsQuiz(2) D(175) Destiny's Child S x + thos.content.blackboardcdn.com/5ebc429344443/2748179?X-Blackboard-Expiration=D1619492400000&X-Blackb... Q ☆ be C Home | Chegg.com My Library Brytew... 6 Introductory Che.. 2 / 2 126% + | A How many mL of a 4 M Al(OH)3 solution are needed to reaction with 125 mL of a 2 M HCl solution? 6. Al(OH)3 (s) + 3 HCl (aq) З НСІ → AICI3 (aq) + 3 H2O (1)arrow_forward
- ECture 1_all.mp4 - Google Drive X A Classes u/0/c/M)Y1MDI1MT95MTM3 lute 50 mlL of the concentratc 00 mL Open with Describe the preparation of 750 mL of 6.00 M H;PO, frc.n the commercial reagent that is 86% H2PO, (w/w) and has a specific gravity of 1.71.arrow_forwardS Test on Balancing | Schoology x K MYKALE MCBROOM-Kami+Ex x S Lab Report no.3: Lesson 1: Wh 1.com/web/viewer.html?document_identifier%3DbljwyGCQs_tEmrlX0nGCWL2C44xHXPP5BAua4FHUTrfdZrdUyzo G iready - Google Sea.. S Warm up Wednesd. a MYKALE MCBROOM - Kami+Export+-+ROSEMARIE+SIBLEY+-+Kami+Export+-+WKS001 002 19 Section 2: Practicing equation balancing Before you can write a balanced equation for a problem which asks you to predict the products of a reaction, you need to know how to balance an equation. Because some of you may not fully remember how to balance an equation, here are some practice problems: 1) C6H6 + O2 > H2O +_ CO2 2) Nal + Pb(SO4)2 → Pbl4 + NazSO4 3) NH3 + O2 →_ NO + H20 4) Fe(OH)3 > Fe2O3 + H20 5) HNO3 + Mg(OH)2 →_H2O + Mg(NO3)2 6) H3PO4 + NaBr → HBr + Na3PO4 7) C+H2 → _ C3H3 8) СаО + Mnl4 . MnO2 + Cal2 9) Fe2O3 + H20 > Fe(OH)з 10) C2H2 +_ H2 → _ C2H6 11) VFs+ HI V2l10 + HF 12) OsO + PtCla PtO + OsCla DELLarrow_forwardHow do you convert 0.485L into mm using unit fractions?arrow_forward
- Answer questions 1 and 2 according to the data given. Dater Weight of cream i: 326 kỳ. Table shows some data obtained directly from Lactascan instrument. Fat % SNF % Lacto- density 27.32 30.23 Raw milk 4.15 7.97 Skim milk 0.08 8.26 Q1. Calculate fat percent of creamn A) 46 B) 28 (C) 3D D) 0.046 آدرما 396 100 x Q2. Calculate efficiency of the cream separator. A) 100% B98% C) 97% D) 96% E) 87.2% amount 2500 L E) 0.28arrow_forwardThe percentage of an additive in gasoline was measured six times with the following results: 0.13; 0.12; 0.16; 0.17; 0.20 and 0.11%. What is the 99% confidence interval for the additive percentage?arrow_forwardCom X Bb Mas X Mas X Hom X K! Kahx Exan X K! Kah X Mitc X КI Kah X K Kah x K! Kah x Kah x K Kahx C session.masteringchemistry.com/myct/itemView?assignmentProblemID=134214560 Apps New Tab Effect of Salinity on... Sleep Disorders | M... P Philo American Journal ... Zeeshan JBAS Ic Wait for Next Quest... oramge juice Effect carrow_forwardQ How X 2 Conv X 3 USCI: X O Login x b Answ x My H x * Minc x * Mind x о Итал х м Почт х * Appli x O NH4 X ng.cengage.com/static/nb/ui/evo/index.html?elSBN=9781305968646&id%3D160085644&snapshotld=456877& CENGAGE MINDTAP Q Search t oter 4: Forces between Particles Note the positive charge; compare with NH4 †. 4.55. C Predict the shape of each of the following polyatomic ions by first drawing a Lewis structure, then applying the VSEPR theory: a. NH2 (each H is bonded to N) b. PO3- (each O is bonded to P) c. BeCl,- (each Cl is bonded to Be) d. ClO4 (each O is bonded to Cl) e d) ENG 144 prt sc delete home 8 9. + backspace num %3D lock K enter Dy Ho P.arrow_forwardO Rich * Ceng x вЫ Еxcel Bb What X O covI x A Daily x O Amon x e Chat x Conte x A ALEK X A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-liJOkWvnm4w-aQ-rw-zRhgRnayfmbBs65spEMBxW_NZIUBEIMJCK O Mail - Ava Schied.. O UAConnect e Biology Syllabus Labflow - Courses S Explore - HogSync e Packback Apps B Blackboard Question 2 Objective Knowledge Check Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: some ionic compounds cation anion empirical formula name of compound Co 3+ so, Co2+ 10, Zn2+ Bro Ba2+ PO I Don't Know Submit O 2020 McGraw-Hi. MacBook Proarrow_forwardme x C Thermo X Cosmet x C Laptops x M Inbox (7 X C Buy TCL X learn.canterbury.ac.uk/ultra/courses/_19016_1/outline/edit/document/_3537106_1?courseld=_19016_1&view=content T K Saturat X ZA a Amazon x C Show Ye X G what's a X Email- 1 X ☆ b The dia x h EX E ct the rato at which + 4. Consider the interconversion of A and B. Suppose in the absence of an enzyme, the forward rate constant KF is 104 s¹ and the reverse rate constant KR is 106 S¹. Calculate the equilibrium constant K. How would the presence of an enzyme affect the value of K? Karrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you

