
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Using the provided ternary phase diagram
1. What is the number of phases in this liquid mixture?
Liquid Amount || Amount (mol)
A || 15
B || 20
C || 7.5
xT || xW || xE
0.5 || 0.25 || 0.25
0.33 ||0.33 || 0.33
0.1 || 0.8 || 0.2
2. What are the phase compositions of the mixtures in Problem 1? What are the trichloromethane-rich and water-rich phases' compositions if there are two phases?
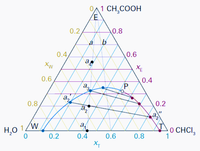
Transcribed Image Text:A1 CH,COOH
0.2
0.8
0.4,
0.6
a
XE
0.6
0.4
0.8
0.2
/W
a,
H,O
0 CHCI,
1
0.2
0.4
0.6
0.8
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- You devise a new temperature scale based on the properties of the liquid propylene glycol, which has a melting point of -59°C and a boiling point of 188°C. Temperature on the new scale will be in °P, where 0.0°P will be correspond to -60.0°C, near the melting point of propylene glycol, and 100°P will correspond to 190.0°C, near the boiling point of propylene glycol. If a substance has a temperature of 49.0°P, what would be its temperature in °C?arrow_forward13. An unknown compound X has the molecular formula C6H140. Compound X shows a strong peak in its IR spectrum at 3000 cm-1. The 1H NMR spectral data of compound X is given below. What is the structure of compound X? absorption triplet singlet quartet singlet ratio 1.0 1.1 6. 1.5 2. 3.5 3. IVarrow_forwardComposition in vapor phase 11 0.8 0.6- Y₁1 0.4 0.2- Equilibrium line 0.6 X₁ Composition in liquid phase 0.2 0.4 0.8 Given the binary mixture above: a) What is the composition in the vapor if the composition in the liquid is x₁=0.6? b) What value is the distribution coefficient at x₁=0.6? c) What value is the selectivity (relative volatility) at x₁=0.6?arrow_forward
- The volume of a liquid varies with temperature according to the following relationship: (³) V = V300K * (0.7 0.75 +3.9 * 10-4 + (²) + + 1.48 * 10-6 where V300K is the liquid's volume at 300K. Calculate the expansion coefficient of this liquid, x, at 320K.arrow_forwardYou are going to prepare a silicone polymer, and one of the starting materials is dichlorodimethylsilane, SiCl2(CH3)2. You need its normal boiling point and to measure equilibrium vapor pressures at various temperatures. What is the normal boiling point of dichlorodimethylsilane? Plot these data as ln P versus 1/T At what temperature does the liquid have an equilibrium vapor pressure of 250 mm Hg? At what temperature is it 650 mm Hg? Calculate the molar enthalpy of vaporization for dichlorodimethylsilane using the Clausius–Clapeyron equation.arrow_forwardI have done 5a, b and C.arrow_forward
- In the first blank, write the coefficient. You must enter this as a number ("1", not "one"). In the second blank, write the chemical formula. Ignore subscripts. For example, Cu(NO3)2 would be entered "Cu(NO3)2". In the third blank, write the state in the following format: (s), (1), (g), or (aq). Make sure you use parenthesis without any spaces! A solution of potassium sulfite reacts with a solution of nitric acid. potassium reactant + acid other product liquid product gas product 个arrow_forwardRequest: Can you please help me with answering the following question? I have provided a diagram to help with the question. Thank you. Question: Given the composition of an iron–carbon alloy containing 0.6 wt% C, at a temperature just below the eutectoid, please specify 1) whether the alloy is hypoeutectoid or hypereutectoid 2) name the proeutectoid phase 3) compute the mass fractions of proeutectoid phase and pearlite 4) make a schematic diagram of the microstructure.arrow_forwardDraw a Pxy diagram for an aqueous solution of NH3. The temperature is such that the vapor pressure of the pure water is 5 kPa and the vapor pressure of pure NH3 is 600 Pa. You need to include the bubble line and the dew 7. line. (a) What is the vapor pressure of the solution when rNH, = 0.6? (b) In which phase the solution will be at P = 4.8 kPa? (c) In which phase the solution will be at P =0.2 kPa? (d) Draw the path for a process that takes a liquid with INH, = 0.6 %3D and P= 4.8 kPa to P= 0.2 kPa.arrow_forward
- The molar absorption coefficients of two substances A and B at two wavelengths (denoted 1 and 2) are as follows: εA1, = 7.5 dm3 mol-1, cm-1, ε B1 = 10.0 dm3 mol-1, cm-1, εA2 = 12.0 dm3 mol-1, cm-1, εB2 = 8.0 dm3 mol-1, cm-1, The total absorbances of a solution at these two wavelengths in a cell oflength 5.0 mm were measured as 0.8 and 1.2. respectively. What are the molar concentrations of A and B in the solution?arrow_forwardComponents A and B are in vapor-liquid equilibrium. Initially, 1 mole of liquid (?? = 0.4) and 0.1 mole of vapor (?? = 0.7) are present. a) What is the overall mole fraction of component A in the system initially? b) 0.5 mole of A is then added and the system goes to equilibrium at the same temperature and pressure. What is the final mole fraction of component A in the system? c) Which of the following statements is correct in achieving the final state? The amount of liquid increases The amount of liquid decreases The concentration of A in the gas phase increases The concentration of A in the liquid phase increasesarrow_forward1 x 104 mole of a substance decomposed when it was irradiated with a light of wavelength 4800 Å. If the quantum efficiency of the reaction is 8, calculate the number of photons absorbed (Avogadro's number = 6.02 × 10²³).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The