
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
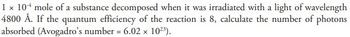
Transcribed Image Text:1 x 104 mole of a substance decomposed when it was irradiated with a light of wavelength
4800 Å. If the quantum efficiency of the reaction is 8, calculate the number of photons
absorbed (Avogadro's number = 6.02 × 10²³).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- The molar absorption coefficients of two substances A and B at two wavelengths (denoted 1 and 2) are as follows: εA1, = 7.5 dm3 mol-1, cm-1, ε B1 = 10.0 dm3 mol-1, cm-1, εA2 = 12.0 dm3 mol-1, cm-1, εB2 = 8.0 dm3 mol-1, cm-1, The total absorbances of a solution at these two wavelengths in a cell oflength 5.0 mm were measured as 0.8 and 1.2. respectively. What are the molar concentrations of A and B in the solution?arrow_forwardPlease answer legibly. What is the meaning of these equations: Langmuir equation and Freundlich equation Please give the meaning of each variable in those two equations. For example: Beer-Lambert Law. A= ϵbC A = absorbance; ϵ = molar absorptivity; b = length of light path; c = concentration. This Beer-Lambert law is just an example of how to do it with the two equations: Langmuir and Freundlich (attached pictures) Also, please include it in the nomenclature. Nomenclature - The symbols should be defined in the nomenclature in alphabetical order. The accompanying definitions must include proper units. Symbol Meaning Unitsarrow_forwardPlz do Asap....!arrow_forward
- Which of the following is true? A Azeotropic mixture is a mixture where the components of vapor phase is higher than the liquid phase and separation at this point can be achieved by using ESA. B Ponchon-Savarit and Mccabe-Thiele method both assumes CMO condition Ponchon-Savarit is more accurate than Mccabe-Thiele method in estimating the number of stages for binary mixtures. D Flash distillation may be used for mixtures having a relative volatility approaching a value of 1arrow_forwardFor a binary mixture distillation column with the following three scenarios, which scenario has its two operating lines coincide with the 45° parity line on the x-y coordinate system? Select one: O A a) O B. b) OcC) OD. None of them.arrow_forwardShort Answer: When you add salt to water, the boiling point is elevated. Briefly connect this to ideal solutions vs non-ideal solutions and mention how inter-molecular forces are involved. If two components were added together and the resulting mixture approximated an ideal solution, what do you expect to be true about the molecular nature of the two components, do you expect them to be similar or dissimilar, why?arrow_forward
- The following figure shows a four-stage separation process for a certain liquid mixture. The ratio P4/D4 is 4, the ratio P3/D3 is 2. the ratio P2/D2 is 1, the ratio of the amount of A to B in stream P3 is 4. a. Calculate the composition and the amount of stream E. Answer: _________of A; ________ of B; _________ of C and __________arrow_forwardsolve as neatly as possible and show completesolution. Round your final answer to four decimal places and box / highlight all final answers. all values must include proper units with properconversion if needed in your solution. kindly follow the format GIVEN REQUIRED SOLUTION THANK YOU! SUBJECT PHYSICAL CHEMISTRYarrow_forwardplease type out or diagrams so that it is easy to readarrow_forward
- Please help me answer this completely with solution ASAParrow_forwardIn an interphase mass transfer operation, the following data were gathered: KG = 0.001 mol/m²-s-Pa k = 0.0005 m/s KG = 4.76x10-5 mol/m2-s-Pa KĻ = 4.76x104 m/s Which of the following statements is true? A) Majority of the mass transfer resistance comes from the gas phase. Both phases equally contribute to the mass transfer resistance. Majority of the mass transfer resistance comes from the liquid phase.arrow_forwardQuestion attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The