
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thank you
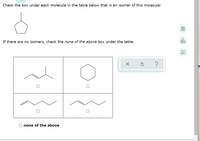
Transcribed Image Text:### Identifying Isomers
In this exercise, you will identify isomers of a given molecule by checking the appropriate boxes. An isomer is a compound with the same chemical formula but a different structural arrangement of atoms.
#### Provided Molecule
The reference molecule is depicted as a six-membered ring with five carbon atoms and one hydrogen atom (cyclopentane).
#### Instruction:
Check the box under each molecule in the table below that is an isomer of this molecule.
If there are no isomers, check the "none of the above" box under the table.
#### Molecule Options
1. **Top Left Molecule**: Contains branching with a double bond.
2. **Top Right Molecule**: Is a six-membered ring similar to the reference molecule (hexane).
3. **Bottom Left Molecule**: Linear molecule with a double bond in the middle.
4. **Bottom Right Molecule**: Linear molecule with a double bond positioned differently compared to the bottom left molecule.
#### Choice:
- **None of the above**
Please check the appropriate boxes to identify which of the molecules are isomers of the given molecule.
### Additional Information
Three options are available on the right side of the page for better navigation:
1. **Calculator Icon**: Likely for performing molecular weight or other calculations.
2. **Graphs Icon**: Possibly provides graphical analysis or additional visual aids.
3. **Table Icon**: Could show tabulated data for better comparison.
Note: The box icons at the bottom of the options likely serve functions such as clearing selections (X), restoring original options (Undo symbol), or providing help (Question mark).
By understanding isomers and their structural differences, you can better appreciate the diversity and specificity of molecular structures in chemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- AutoSave w Cinnamates DIR - Compatibility Mode OFF Home Insert Draw Design Layout References Mailings Review View Table Design Layout Tell me Share O Comments Times New... v 12 A A Аa v AaBbCcD AaBbCcDdE AaBb AаBbCcDdEe AaBbCcDdEe AaBbCcDdE E AaBbCcDdEe AaBbCcDdEe AaBbCcDdEe > Paste A • ev A No Spacing Title Subtle Emph... Normal Heading 1 Heading 2 Subtitle Emphasis Intense Emp.. Styles Pane B U v ab x, Dictate Sensitivity trans-cinnamic acid Work up your spectrum well, and fill in Table 1 below. Omit any impurities, solvents, or other elements that are not part of your compound. For the Figure, make a good drawing in ChemDraw (ACS-1996 settings), inserting the compound name in bold below the structure. Then using small, bold, lowercase letters to match the table, assign all of the 'H signals. Figure 1: Structure and 'H NMR assignments for trans-cinnamic acid. ОН Table 1: Experimental 'H NMR data for trans-cinnamic acid. Signal 8 (ppm) Mult. J-values (Hz) Int. a 11.3 1H b 1H 2H d 3H е…arrow_forwardBOROSILICATE 600 Supertek 600ml BOROSILICATE GLASS 3.3 500 400 300 200 100 APPROX. VOLarrow_forwardA ALEKS - Dona Luc - Lean H My Grades - 2021 Spring Term (2 X Tutor.com Learning Suite M Hey - lucdona7@gmail.com - Gm x www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IBIZZhveDw7yX8A9043nt5P1XWJwAREDsbwIERg1UdvpRqH651Jk. O MATTER Solving applied density problems Mr. Auric Goldfinger, criminal mastermind, intends to smuggle several tons of gold across international borders by disguising it as lumps of iron ore. He commands his engineer minions to form the gold into little spheres with a diameter of exactly 6 cm and paint them black. However, his chief engineer points out that customs officials will surely notice the unusual weight of the "iron 3. ore" if the balls are made of solid gold (density 19.3 g/cm). He suggests forming the gold into hollow balls 3. instead (see sketch at right), so that the fake "iron ore" has the same density as real iron ore (5.15 g/cm). One of the balls of fake "iron ore," sliced in half. Calculate the required thickness of the walls of each hollow…arrow_forward
- Answer provided: 2.5% Al2(SO4)3 Please show your complete solution and write your answer clearly and readable. Thank you.arrow_forwardin text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forwardSodium (Na) is a shiny silver-coloured metal at room temperature. Zinc is also a shiny silver-coloured metal at room temperature. If sodium and zinc were placed in front of you, unlabelled, how would be able to tell which metal is which? (Hint: You may wish to consider the properties of these two metals.)arrow_forward
- The inital points are ( 0, 27.12) and (0.00250 , 5.17)arrow_forwardcollege.com/course.htm MS-¡PI... Give the correct IUPAC name for each of the following compounds. Submit Part C Request Answer CH₂ CH₂ CH₂ CH3 CH3CHCH₂CH CH3 CH₂CH3 Spell out the full name of the compound. Submit Request Answer P Pearson Review | Constants | Periodic Terms of Use | Privacy Policy | Permissions Contact Us Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use O 2 D 9:26 1 10/14/20arrow_forwardA Homework and quiz in CHEM101 X My Questions | bartleby M Inbox (2) - comicnerd150@gmail X M Inbox (1) - brianfaust150@gmail. X + A learn.maricopa.edu/courses/1132256/modules/items/19018031 CHM151 17003 > Modules> Weeks 8 & 9 - Chapter 7 > Homework and quiz in CHEM101 - due Sunday night CG 2020 FALL CRED Question 20 of 30 Submit Account Home Complete the balanced neutralization equation for the reaction below: Announcements Dashboa |Modules коНfaq) + HaS0-(aq) — rd Concourse Syllabus Courses Grades D2+ 13+ 4+ Cisco Webex Groups 1 2 3 4 7 8 9. Tutoring/Learning Center Calendar Os Do Inbox (1) (g) (aq) History H K Help • Previous Next » Library 6:55 PM O Type here to search 10/21/2020 1Larrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY