
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:All changes saved
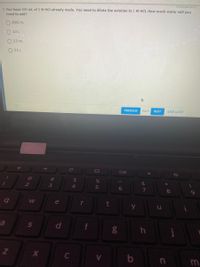
3. You have 500 mL of 5 M Hcl already made. You need to dilute the solution to 1 M HCI. How much water will you
need to add?
2500 mL
2.0 L
O 2.5 mL
O 2.5 L
PREVIOUS
3 of 5
NEXT
SAVE & EXIT
23
24
41
%
2
3
&
6
7
8
e
a
d
f
V
m
0.0
ST
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- URGENT PLEASE ANSWER CORRECTLY THANK YOUarrow_forwardHello, A solution contains 0.350 grams of malonic acid, H2C3H2O4 in 500.0 mL. (Malonic acid a diprotic acid). What is the % (w/v) of the solution? Thank you,arrow_forwardWhat is the concentration of an HNO3 solution in percent by mass that was determined to have a concentration of 0.669 M in a titration experiment? Assume a density of 1.00 g/mL. Your Answer: Answer units Previous Page Next Page Page 4 of 15arrow_forward
- You want to prepare a 100ml of 2M Barium hydroxide and you only have 9M barium hydroxide. How much should you use?arrow_forwardHello sir, I just want a solution without explanations and a clear linearrow_forwardFor the questions of Exp. 13, Calculate the Molarity (M) of 30.87 mL NaOH solution when it is titrated with 0.332 g of sulfamic acid (Mw = 97.10 g/mol). Type your answer...arrow_forward
- Given the following value of H3O" |, calculate the corresponding value of [OH] for each solution. a. H3 O =1 × 10 -² M [OH ] = M b. [H2O*] =1: 1 × 10-9 M 6-1 M = [_HO]arrow_forwardWhat is the concentration of H* in a 2.03 M HCI solution? O 2.03 M O 1.0 x 10-14 M 02.03 M O not enough information Onone of the above Warrow_forwardHow do you caclulate the water added?arrow_forward
- Nonearrow_forwardWhat is an acid-base reaction? Match the items in the left column to the appropriate blanks in the sentences on the right. a precipitate a new acid or a new base OH- H₂O(1) H+ When an acid and base are mixed, the from the base to form from the acid combines with the Reset Helparrow_forward||| ctrl lacc shift ↑ tab caps lock VILION Mc ALE Graw HI esc K →1 Pavilion x360 fn O CHEMICAL REACTIONS Determining the molar mass of an acid by titration McCA X Eplanation g mol V An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then titrates this solution with 0.1500M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 38.4 mL of NaOH solution. Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits. f1 Type here to search A https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym f2 @ Z Check 2 L M W hp S ALE MCCA ALER f3 # Mc Graw 3 X alt D 4 $ 4 с x10 X R O f5 Me Graw MI % F 5 at 40 MCCA ALE ALE ● V T S f6 4- G 6 ■ T f7 B Y ♫+ & Mc 7 H raw fg MCCA ALER *. N KAA © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY