
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Problem 4.1
Transcribed Image Text:Sun 4:07 PM
Karla De Los Santos
P So X
openvellum.ecollege.com/course.html? courseld=15512856&HepID=5e6f971 37bf41908275b6c5ec1 c1 fc
iCloud Storage is Full
Upgrade your storage to keep
using iCloud.
UP
nistry
Course Home
NO
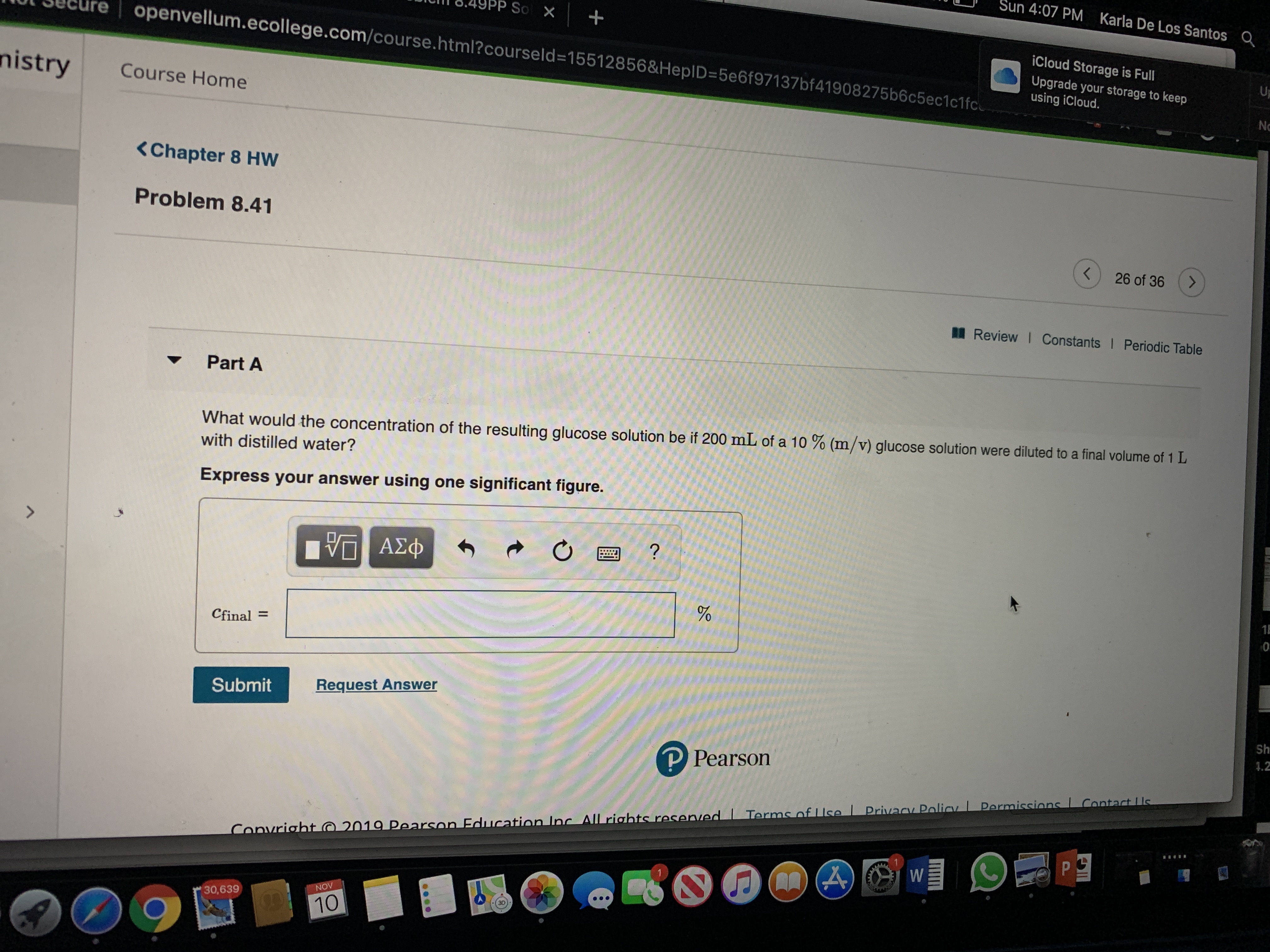
<Chapter 8 HW
Problem 8.41
26 of 36
Review I Constants I Periodic Table
Part A
What would the concentration of the resulting glucose solution be if 200 mL of a 10 % (m/v) glucose solution were diluted to a final volume of 1 L
with distilled water?
Express your answer using one significant figure.
ΑΣΦ
1E
0
Cfinal=
Request Answer
Submit
Sh
1.2
P Pearson
Contact Us
Permissins
Privacy Poliay
2019 Pearson Fducation Lnc All rights reservedI Terms nf Use
Convriaht
1
NOV
30,639
3D
10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- B 0C1- Assignment 4 - Chapter 4- x S WileyPLUS A edugen.wileyplus.com/edugen/Iti/main.uni WileyPLUS Brown, Intro Organic Chemistry, 6e Help I System Announcements ASSIGNMENT RESOURCES PRINTER VERSION Explain why each name is incorrect, and then write a correct name for the intended compound: OC1- Assignment 4 - Chapter 4 Problem 4.32 Problem 4.22 Problem 4.20 O Problem 4.42 O Practice Question 04 O Practice Question 06 O Problem 4.14 Problem 4.36 Problem 4.10 E Practice Question 09 Practice Question 07 O Practice Question 05 O Problem 4.30 O Problem 4.34 (a) 2-Ethyl-1-propene The name is incorrect because The correct IUPAC name is (b) 5-Isopropylcyclohexene The name is incorrect because The correct IUPAC name is (c) 4-Methyl-4-hexene The name is incorrect because The correct IUPAC name is Review Score Review Results by Study Objective (d) 2-sec-Butyl-1-butene The name is incorrect because The correct IUPAC name is (e) 6,6-Dimethylcyclohexene The name is incorrect because The correct…arrow_forwardFlock Browser Guard™ Provide a multi-step synthesis of the "Final Product from the given "Reactant" by completing the synthesis scheme below. Draw the entire synthesis scheme & complete the synthesis scheme and box your answers as shown below. Indicate the set of reagents/conditions #1, #2, #3, #4; and Draw the chemical structure of major organic product at each step i.e. Compounds A, B, and C. Each set of reagents/conditions may contain more than one reagent. Use the notation 1., 2., etc., to show the steps in each set of reagents/conditions as appropriate. Do not show any curved- arrow pushing mechanisms. Q Search $ - 1163 110 Il app.honorlock.com is sharing you 99+arrow_forwardExample 4 Example 24 Example 1* Please indicate with an arrow and draw a three-dimensional picture to explain. Example 14 C C H H CO₂CH3 CO₂CH3 NaCl Br 160 °C toluene OTMS OTMS Br Cope 重 Krapcho wet DMSO reflux Br 71%arrow_forward
- Aa.7.arrow_forward6) Which products are, respectively, the kinetic and thermodynamic product? Br Br Br HBr (1 equiv.) 1. 2. 3.arrow_forwardVol 7.80 LTE KB/s 59 47:18 0 expert.chegg.com [1] Show me the steps to solve everything in yellow in the attached image with the excel formulas. Given TUM Petroleum Cost of Goods Sold Gross Pr Dec-14 Dec-15 $12.211.00 $13.368.00 (9,75600) (10,591.00) 245600 2.777.00 Selling General & Administrative Expen Operating Income Before Deprec 1704.00) 1698.00) 2.079.00 Depreciation, Depletion, & Amorázation Cowrating Pront Test Expense Non-Operating income Expense Special em $500 128) 139.00 2000 1200 1200 151.00 Prefa Income 1,064.00 Total Income Tax (34080) 142560) Net Income S205 40 Purchase of PP&E CAPEX) 815 1.322 Increase in Not Working Capital 102 14:30) TCMɛ rate Solution a. FCF Calculators for 2014-20 2014 2015 EBIT (Operating Prof EBITIT NOPAT LE CAPEX Plus Depreciation Exper Lase: Working Capital Investment Firm Free Cash Flow b. Estimated FCF for 2016-2020 EBIT (Growing 100% per year) EBIT-40-NOPAT Plus Depreciation Expense L CAPEX Less: Working Capital Im Firm Free Cash Flow…arrow_forward
- Please make sure you draw it in a format that is able to be recreated in the hexagons shown in picture!arrow_forward12:49 1 Send a chat Paragraph Styles Edit I 1 2 . 3 4 L..5 . 6.. I 7.. Problems from Periods 18-19: 1. Gasoline combustion engines run on the following balanced chemical reaction between octane (CH) and oxygen (0.): 20,H1 (g) + 250, (g) 16CO, (g) 18H,0 (g) A small car's gas tank holds 34292 grams (75.6 |bs) of gasoline. a. How many grams of the greenhouse gas CO, are produced by one tank of gasoline? Convert that number to pounds (Ibs). b. How many grams of O, are necessary to completely react with one tank of gasoline? CPacusarrow_forward5.9. In the polymerization of H2N(CH2 )10 COOH to form nylon-11, what is the molecular weight of the species that has the largest weight fraction in the reaction mixture at 99% conversion ? [Ans. 18,300]arrow_forward
- +|| V Σ 00 What is the theoretical mole ratio of sodium carbonate deca-hydrate to sodium chloride? O 1:2 O 1:1 O 2:1 MacBook Air 08 F3 000 000 F1 888 F4 DD F7 F5 F6 (D F11 F8 # OLJ F12 $ & * 3. 5. 9. 8. 6. delete R } { H. retu B. N command optionarrow_forward2.2.1 Gene Thera X O0 Inbox (9,416) - va x b My Questions | ba x Things I Can't For X Course Home + USC A openvellum.ecollege.com/course.html?courseld=16196689&HepID=aeb74d6324e98a415a0c4c5c32b9c2e6#10001 Mooney AP Chemistry 20-21 Sign Out Help Hi, Vari v Mastering Chemistry Course Home I Review | Constants | Periodic Table MISSED THIS? Read Section о Му Сourses 16.8 (Pages 701 - 710) ; Watch KCV 16.8, IWE 16.9. Part A Course Home For the following reaction, Kc = 255 at 1000 K. CO (g) + Cl2 (g) = COC12 (g) If a reaction mixture initially contains a CO concentration of 0.1470 M and a Cl2 concentration of 0.174 M at 1000 K. What is the equilibrium concentration of CO at 1000 K? Syllabus Scores Express your answer in molarity to three significant figures. • View Available Hint(s) Pearson eText Study Area Document Sharing [CO] = M User Settings Submit Course Tools > Part B What is the equilibrium concentration of Cl2 at 1000 K? Express vour answer in molarity to three significant figures.…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY