
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
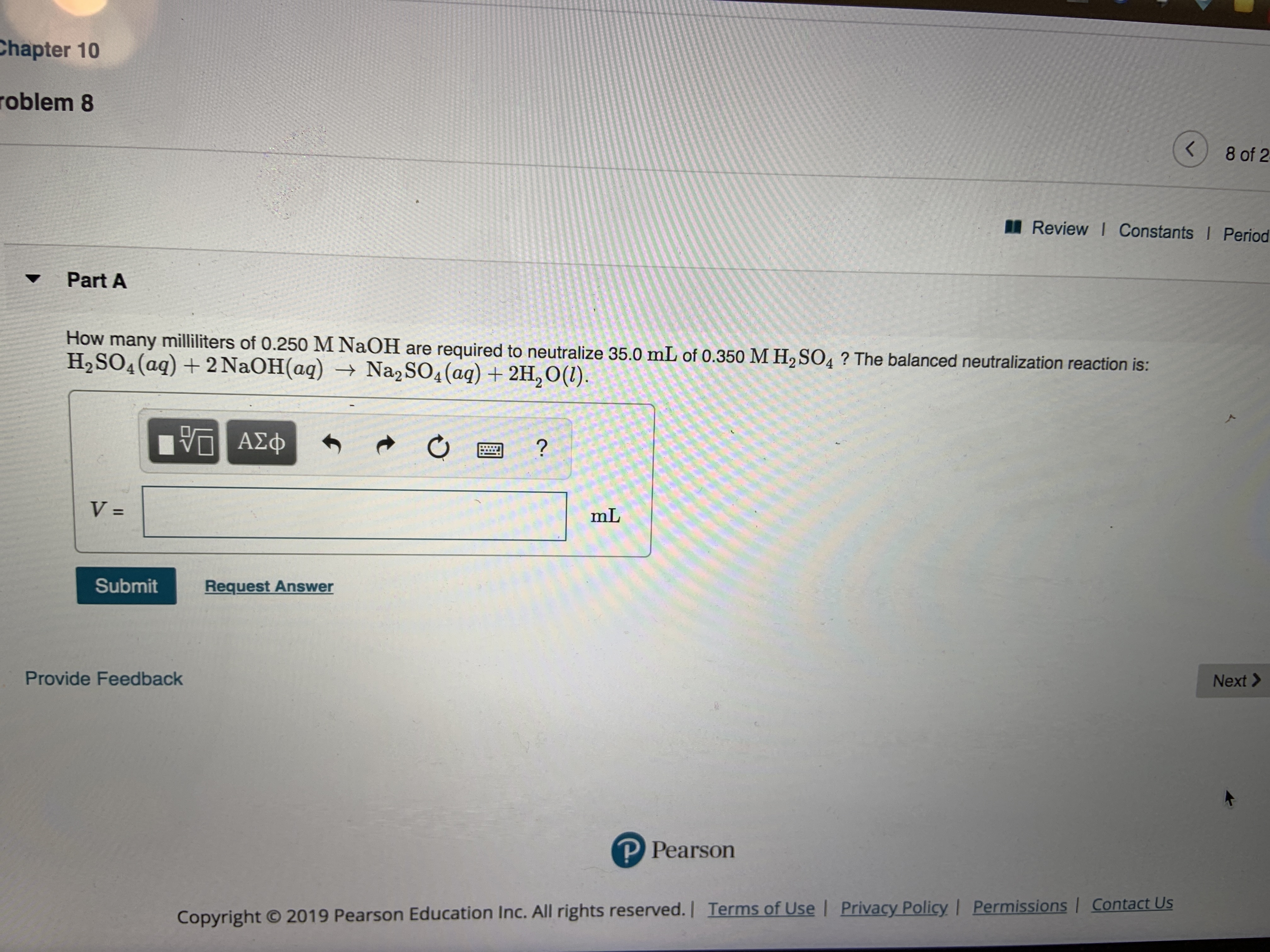
Transcribed Image Text:Chapter 10
roblem 8
8 of 2
Review I Constants Period
Part A
How many milliliters of 0.250 M NaOH are required to neutralize 35.0 mL of 0.350 M H2 SO4? The balanced neutralization reaction is:
H2SO4(aq)+2 NaOH(aq) Na2 SO4(aq)2H,O(1)
VOAED
?
V =
mL
Submit
Request Answer
Provide Feedback
Next>
P Pearson
Contact Us
Permissions
Privacy Policy
Copyright 2019 Pearson Education Inc. All rights reserved.| Terms of Use
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- When 85.0 mL of 0.250 M Ba(OH)2 solution is added to 85.00 mL of 0.250 M Al (NO3)3 solution, a white gelatinous precipitate of Al(OH)3; is formed. Assuming 100% yield, (a) what mass (in grams) of Al(OH)3 is formed? (b) what is the molarity of each of the ions Ba2+, OH-, Al3+, NO3- in the resulting solution?arrow_forwardCalcium carbonate, CaCO3, can be obtained in a very pure state. Standard solutions of calcium ion are usually prepared by dissolving calcium carbonate in acid. What mass of CaCO3 should be taken to prepare 500. mL of 0.0200 M calcium ion solution?arrow_forwardWhat volume of 0.250 M NaOH is required to react completely with 0.0100 moles of H2SO4? (a) 80.0 mL (b) 60.0 mL (c) 40.0 mL (d) 125 mLarrow_forward
- 60. Suppose 325 in L of 0.150 M NaOH is needed for your experiment. How would you prepare this if all that is available is a 1.01 M NaOH solution?arrow_forward68. Aluminum ion may be precipitated from aqueous solution by addition of hydroxide ion, forming Al(OH)3. A large excess of hydroxide ion must not be added, however, because the precipitate of Al(OH)3 will redissolve as a soluble compound containing aluminum ions and hydroxide ions begins to form. How many grains of solid NaOH should be added to 10.0 mL of 0.250 M A1Cl3 to just precipitate all the aluminum?arrow_forward87. What volume of 0.151 N NaOH is required to neutralize 24.2 mL of 0.125 N H2SO4? What volume of 0.151 N NaOH is required to neutralize 24.2 n1L of 0.125 M H2SO4?arrow_forward
- The molarity of iodine in solution can be determined by titration with arsenious acid, H3AsO4. The unbalanced equation for the reaction is H3AsO3(aq)+I2(aq)+H2O2 I(aq)+H3AsO4(aq)+2 H+(aq)A 243-mL solution of aqueous iodine is prepared by dissolving iodine crystals in water. A fifty-mL portion of the solution requires 15.42 mL of 0.134 M H3AsO3 for complete reaction. What is the molarity of the solution? How many grams of iodine were added to the solution?arrow_forwardVitamin C has the formula C6H8O6. Besides being an acid, it is a reducing agent. One method for determining the amount of vitamin C in a sample is to titrate it with a solution of bromine, Br2, an oxidizing agent. C6H8O6(aq) + Br2(aq) 2 HBr(aq) + C6H6O6(aq) A 1.00-g "chewable" vitamin C tablet requires 27.85 ml of 0.102 M Br2 for titration to the equivalence point. What is the mass of vitamin C in the tablet?arrow_forward3.85 The particulate drawing shown represents an aqueous so- lution of an acid HA, where A might represent an atom or group of atoms. Is HA a strong acid or a weak acid? Explain how you can tell from the picture.arrow_forward
- According to the Resource Conservation and Recovery Act (RCRA), waste material is classified as toxic and must be handled as hazardous if the lead concentration exceeds 5 mg/L. By adding chloride ion, the lead ion will precipitate as PbCl2, which can be separated from the liquid portion. Once the lead has been removed, the rest of the waste can be sent to a conventional waste treatment facility. How many grams of sodium chloride must be added to 500 L of a waste solution to reduce the concentration of the Pb2+ ion from 10 to 5 mg/L?arrow_forwardssume a highly magnified view of a solution of HCI that allows you to “see” the HCl. Draw this magnified view. If you dropped in a piece of magnesium, the magnesium would disappear, and hydrogen gas would he released. Represent this change using symbols for the elements, and write the balanced equation.arrow_forwardSilver ions can be found in some of the city water piped into homes. The average concentration of silver ions in city water is 0.028 ppm. (a) How many milligrams of silver ions would you ingest daily if you drank eight glasses (eight oz/glass) of city water daily? (b) How many liters of city water are required to recover 1.00 g of silver chemically?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning